正在加载图片...
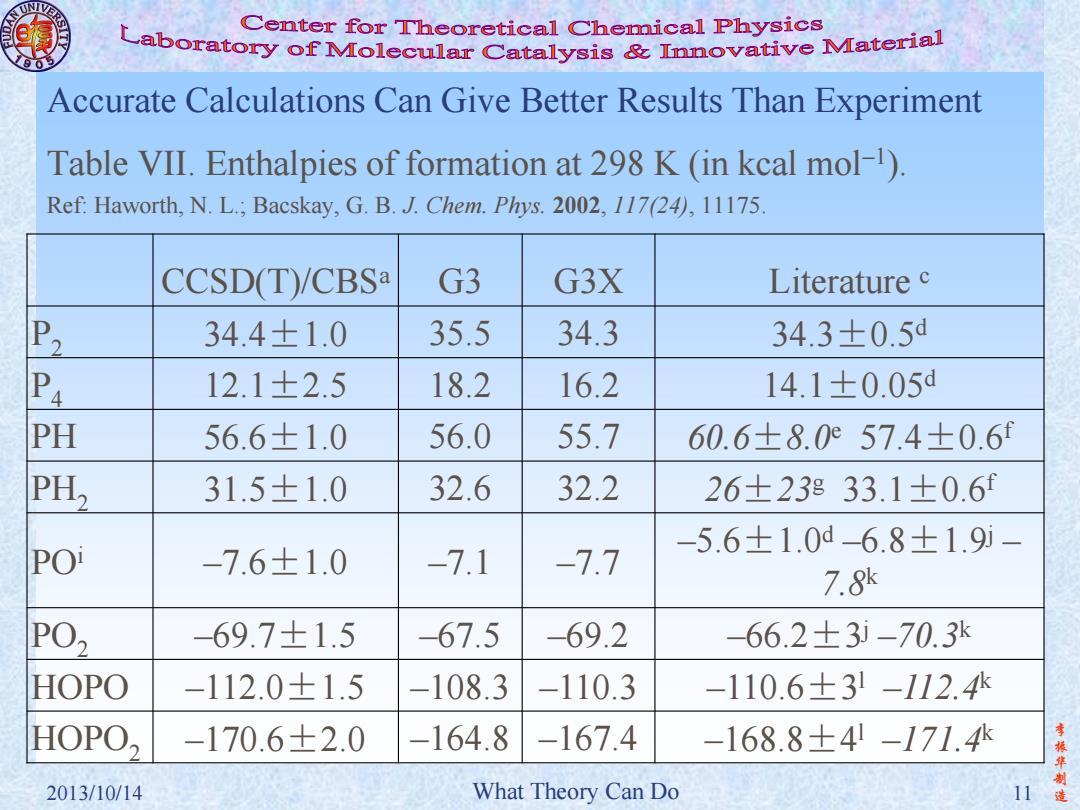
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis mvative Material Accurate Calculations Can Give Better Results Than Experiment Table VII.Enthalpies of formation at 298 K (in kcal mol-1). Ref:Haworth,N.L.;Bacskay,G.B.J.Chem.Phys.2002,117(24),11175. CCSD(T)/CBSa G3 G3X Literature c P2 34.4±1.0 35.5 34.3 34.3±0.5d P4 12.1±2.5 18.2 16.2 14.1±0.05d PH 56.6±1.0 56.0 55.7 60.6±8.057.4±0.6f PH, 31.5±1.0 32.6 32.2 26±23833.1±0.6f -5.6±1.0d6.8±1.9i- POi -7.6±1.0 -7.1 -7.7 7.8 PO, -69.7±1.5 -67.5 -69.2 -66.2±3i-70.3k HOPO -112.0±1.5 -108.3 -110.3 -110.6±31-112.4 HOPO, -170.6±2.0 -164.8 -167.4 -168.8±4-171.4 李 2013/10/14 What Theory Can Do 11造李 振 华 制 2013/10/14 What Theory Can Do 11 造 Accurate Calculations Can Give Better Results Than Experiment CCSD(T)/CBSa G3 G3X Literature c P2 34.4±1.0 35.5 34.3 34.3±0.5d P4 12.1±2.5 18.2 16.2 14.1±0.05d PH 56.6±1.0 56.0 55.7 60.6±8.0e 57.4±0.6f PH2 31.5±1.0 32.6 32.2 26±23g 33.1±0.6f POi –7.6±1.0 –7.1 –7.7 –5.6±1.0d –6.8±1.9j – 7.8k PO2 –69.7±1.5 –67.5 –69.2 –66.2±3 j –70.3k HOPO –112.0±1.5 –108.3 –110.3 –110.6±3 l –112.4k HOPO2 –170.6±2.0 –164.8 –167.4 –168.8±4 l –171.4k Table VII. Enthalpies of formation at 298 K (in kcal mol–1 ). Ref: Haworth, N. L.; Bacskay, G. B. J. Chem. Phys. 2002, 117(24), 11175