正在加载图片...
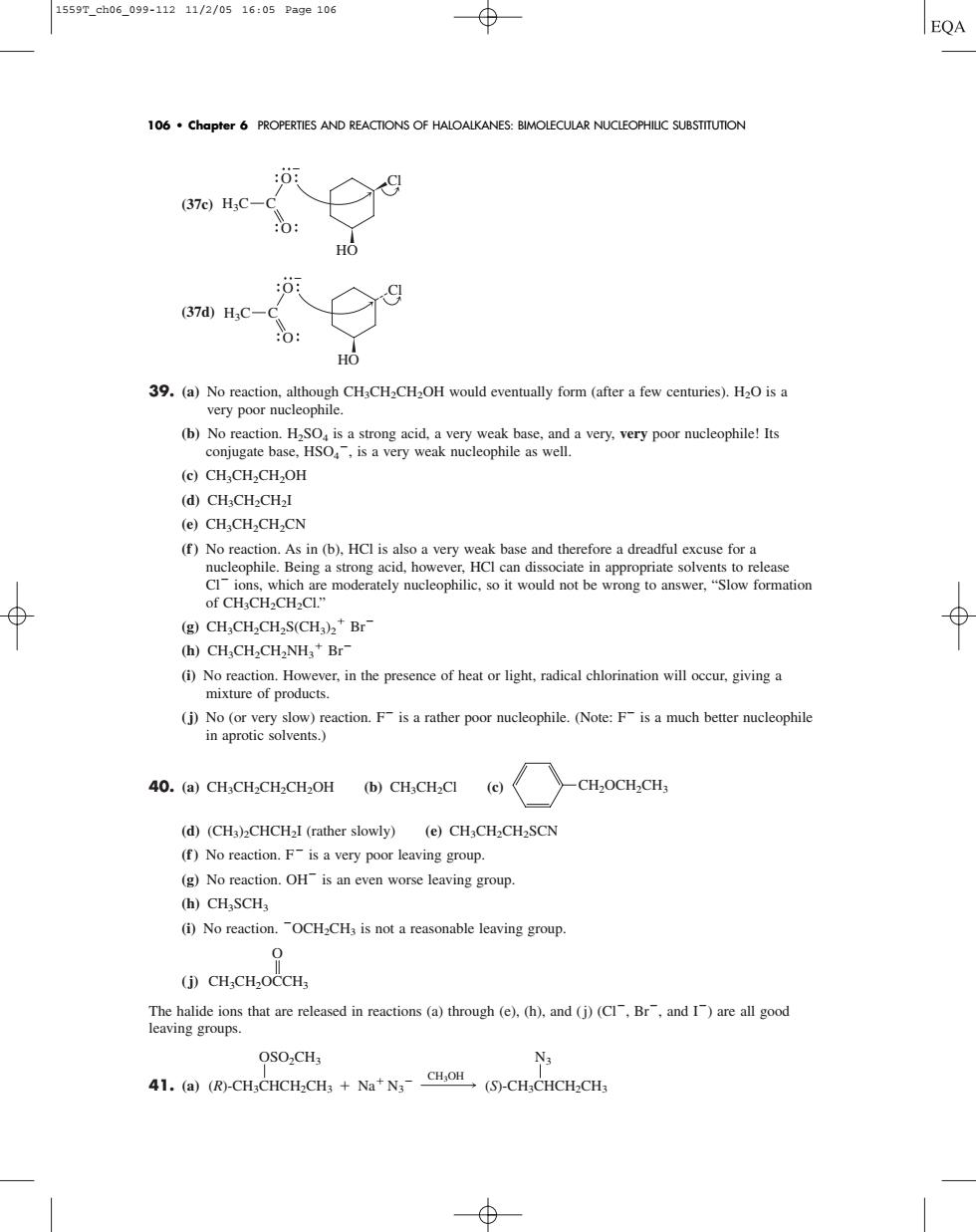
1559T_ch06_099-11211/2/0516:05Pa9e106 ⊕ EQA 106.Chapter 6 PROPERTES AND REACTIONS OF HALOALKANES:BIMOLECULAR NUCLEOPHILIC SUBSTITUTION (37e)H:C-C 9 39.(a)No reaction,although CH3CH2CH2OH would eventually form(after a few centuries).H2O is a very poor nucleophile. (b)No reaction.HSO.is a st acid.a ver weak base,and a very,very poor nucleophile!Its conjugate base.HSOis a very weak nucleophile as well. (e)CHCH2CH2OH (d)CH;CH2CH2I (e)CH.CH.CH.CN use for (g)CH:CH,CH2S(CH)Br (h)CH,CH2CH2NH,*Br- No reaction..Ho (j)No (or y 40.(a)CH:CH2CH2CH2OH (b)CH:CH2CI -CH2OCH2CH, (d)(CHCHCHI (rather slowly) (e)Chch-CH-SCN (f)No reaction.F-isa very poor leaving group. (g)No reaction.OH is an even worse leaving group (h)CHSCH (i)No reaction.OCHCH,is not a reasonable leaving group. (CH.CH.OCCH OSO2CH3 N3 41.(a)(R-CHCHCH.CH+Na'N (5)-CH CHCH.CH (37c) (37d) 39. (a) No reaction, although CH3CH2CH2OH would eventually form (after a few centuries). H2O is a very poor nucleophile. (b) No reaction. H2SO4 is a strong acid, a very weak base, and a very, very poor nucleophile! Its conjugate base, HSO4 , is a very weak nucleophile as well. (c) CH3CH2CH2OH (d) CH3CH2CH2I (e) CH3CH2CH2CN (f ) No reaction. As in (b), HCl is also a very weak base and therefore a dreadful excuse for a nucleophile. Being a strong acid, however, HCl can dissociate in appropriate solvents to release Cl ions, which are moderately nucleophilic, so it would not be wrong to answer, “Slow formation of CH3CH2CH2Cl.” (g) CH3CH2CH2S(CH3)2 Br (h) CH3CH2CH2NH3 Br (i) No reaction. However, in the presence of heat or light, radical chlorination will occur, giving a mixture of products. (j) No (or very slow) reaction. F is a rather poor nucleophile. (Note: F is a much better nucleophile in aprotic solvents.) 40. (a) CH3CH2CH2CH2OH (b) CH3CH2Cl (c) (d) (CH3)2CHCH2I (rather slowly) (e) CH3CH2CH2SCN (f ) No reaction. F is a very poor leaving group. (g) No reaction. OH is an even worse leaving group. (h) CH3SCH3 (i) No reaction. OCH2CH3 is not a reasonable leaving group. (j) The halide ions that are released in reactions (a) through (e), (h), and (j) (Cl, Br, and I) are all good leaving groups. 41. (a) (R)-CH3CHCH2CH3 (S)-CH3CHCH2CH3 OSO2CH3 Na N3 N3 CH3OH CH3CH2OCCH3 O CH2OCH2CH3 H3C C O O Cl HO H3C C O O Cl HO 106 • Chapter 6 PROPERTIES AND REACTIONS OF HALOALKANES: BIMOLECULAR NUCLEOPHILIC SUBSTITUTION 1559T_ch06_099-112 11/2/05 16:05 Page 106����