正在加载图片...
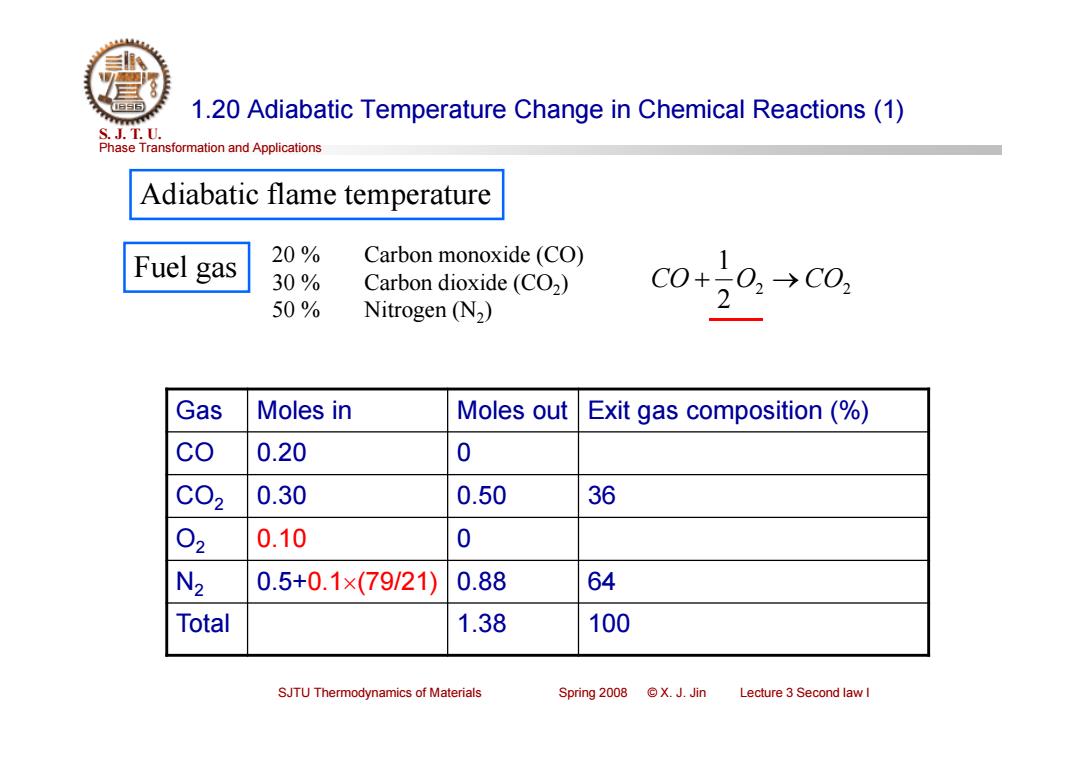
1.20 Adiabatic Temperature Change in Chemical Reactions(1) S.J.T.0. Phase Transformation and Applications Adiabatic flame temperature Fuel gas 20% Carbon monoxide (CO) 30% Carbon dioxide (CO2) 50% C0+20,→c0, Nitrogen (N2) 2 Gas Moles in Moles out Exit gas composition (% co 0.20 0 C02 0.30 0.50 36 02 0.10 0 N2 0.5+0.1×(79/21) 0.88 64 Total 1.38 100 SJTU Thermodynamics of Materials Spring2008©X.J.Jin Lecture 3 Second law IPhase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2008 © X. J. Jin Lecture 3 Second law I 1.20 Adiabatic Temperature Change in Chemical Reactions (1) Adiabatic flame temperature Gas Moles in Moles out Exit gas composition (%) CO 0.20 0 CO2 0.30 0.50 36 O2 0.10 0 N2 0.5+0.1(79/21) 0.88 64 Total 1.38 100 Fuel gas 20 % Carbon monoxide (CO) 30 % Carbon dioxide (CO2) 50 % Nitrogen (N2) 2 2 2 1 CO O CO