正在加载图片...
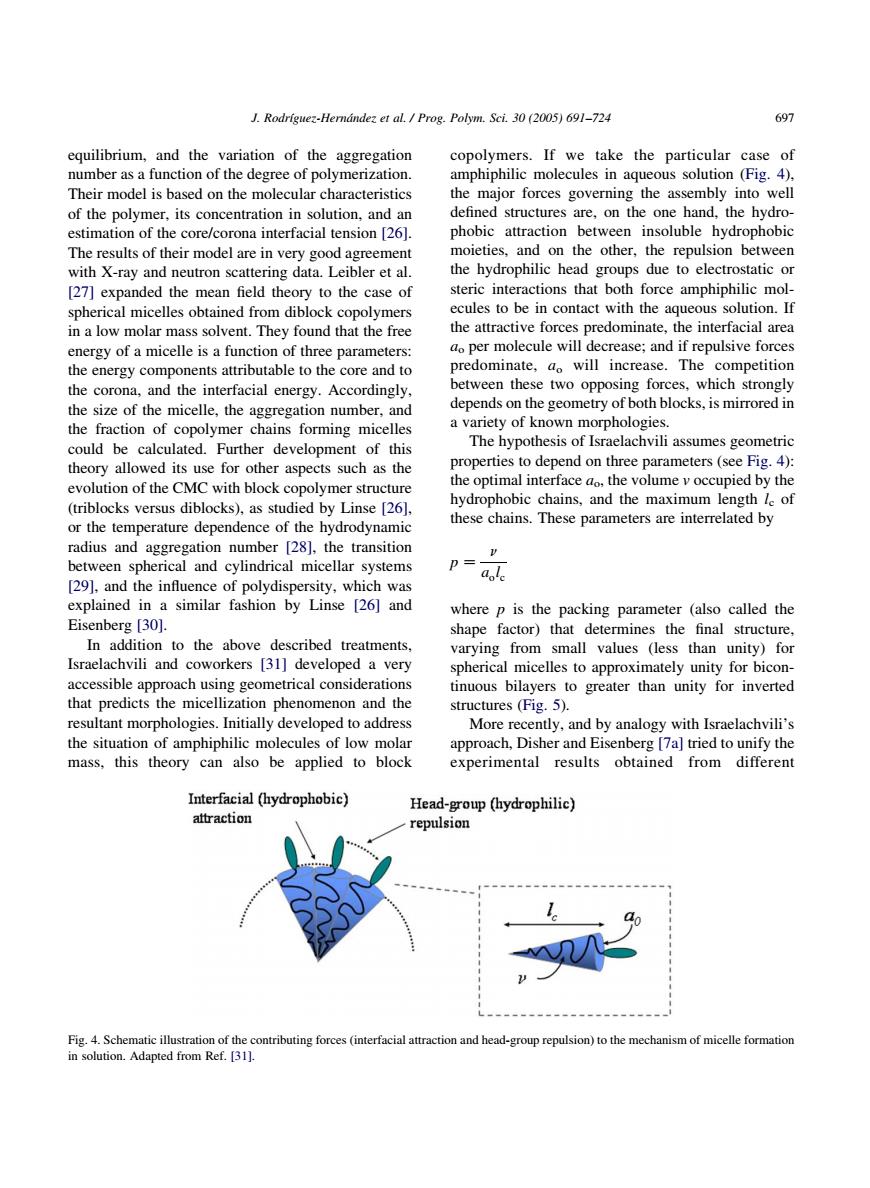
J.Rodriguez-Hemdndezet al /Prog.Polym.Sci.3(005)691-724 697 equilibrium.and the variation of the aggregation copolymers.If we take the particular case of number as a function of the degree of polymerization. amphiphilic molecules in aqueous solution (Fig.4). Their model is based on the molecular characteristics the major forces governing the assembly into well of the polymer,its concentration in solution,and an defined structures are,on the one hand,the hydro estimation of the core/corona interfacial tension 1261. attraction between soluble hydrophobi The results of their model are in very good agreement the hy dophi on the othe on be with X-ray and neutron scattering data.Leibler et al. e o le [27]expanded the mean field theory to the case of with th eamphiphili the attractive forc ate the inte facial are per molecule will decrease and if repulsive force e parame predominate,a will increase.The competition cnergy con core and the between these two opposing forces.which strongly g depends on the geometry of both blocks,is mirrored in va ,ofhi s sume such as the ers (see evolution of the cMC with block co olymer structure (triblocks versus diblocks).as studied by Linse [26]. hobic chains.and the ngth le of or the temperature dependence of the hydrodynamic these chains.These parameters are interrelated by radius and aggregation number [28],the transition between spherical and cylindrical micellar systems p= 29,and the influence of polydispersity,which was explained in a similar fashion by Linse [26]and where p is the packing parameter (also called the Eisenberg [30]. shape factor)that determines the final structure. In addition to the above described treatments varying from small values (less than unity)for and rkers [31]developec spherical micelles to approximately unity for bicon- appro ncal nd the tinuous bilayers to greater than unity for inverted structures(Fig.5), More hinhilic this theory can also be applied to block app obtained from 、dif rer Interfacial (hydrophobic) fesctrhaRftinhaiagaroesaatcaanrtoandheadgopepukiootemectanmofmoeeamio equilibrium, and the variation of the aggregation number as a function of the degree of polymerization. Their model is based on the molecular characteristics of the polymer, its concentration in solution, and an estimation of the core/corona interfacial tension [26]. The results of their model are in very good agreement with X-ray and neutron scattering data. Leibler et al. [27] expanded the mean field theory to the case of spherical micelles obtained from diblock copolymers in a low molar mass solvent. They found that the free energy of a micelle is a function of three parameters: the energy components attributable to the core and to the corona, and the interfacial energy. Accordingly, the size of the micelle, the aggregation number, and the fraction of copolymer chains forming micelles could be calculated. Further development of this theory allowed its use for other aspects such as the evolution of the CMC with block copolymer structure (triblocks versus diblocks), as studied by Linse [26], or the temperature dependence of the hydrodynamic radius and aggregation number [28], the transition between spherical and cylindrical micellar systems [29], and the influence of polydispersity, which was explained in a similar fashion by Linse [26] and Eisenberg [30]. In addition to the above described treatments, Israelachvili and coworkers [31] developed a very accessible approach using geometrical considerations that predicts the micellization phenomenon and the resultant morphologies. Initially developed to address the situation of amphiphilic molecules of low molar mass, this theory can also be applied to block copolymers. If we take the particular case of amphiphilic molecules in aqueous solution (Fig. 4), the major forces governing the assembly into well defined structures are, on the one hand, the hydrophobic attraction between insoluble hydrophobic moieties, and on the other, the repulsion between the hydrophilic head groups due to electrostatic or steric interactions that both force amphiphilic molecules to be in contact with the aqueous solution. If the attractive forces predominate, the interfacial area ao per molecule will decrease; and if repulsive forces predominate, ao will increase. The competition between these two opposing forces, which strongly depends on the geometry of both blocks, is mirrored in a variety of known morphologies. The hypothesis of Israelachvili assumes geometric properties to depend on three parameters (see Fig. 4): the optimal interface ao, the volume v occupied by the hydrophobic chains, and the maximum length lc of these chains. These parameters are interrelated by p Z n aolc where p is the packing parameter (also called the shape factor) that determines the final structure, varying from small values (less than unity) for spherical micelles to approximately unity for bicontinuous bilayers to greater than unity for inverted structures (Fig. 5). More recently, and by analogy with Israelachvili’s approach, Disher and Eisenberg [7a] tried to unify the experimental results obtained from different Fig. 4. Schematic illustration of the contributing forces (interfacial attraction and head-group repulsion) to the mechanism of micelle formation in solution. Adapted from Ref. [31]. J. Rodrı´guez-Herna´ndez et al. / Prog. Polym. Sci. 30 (2005) 691–724 697