正在加载图片...
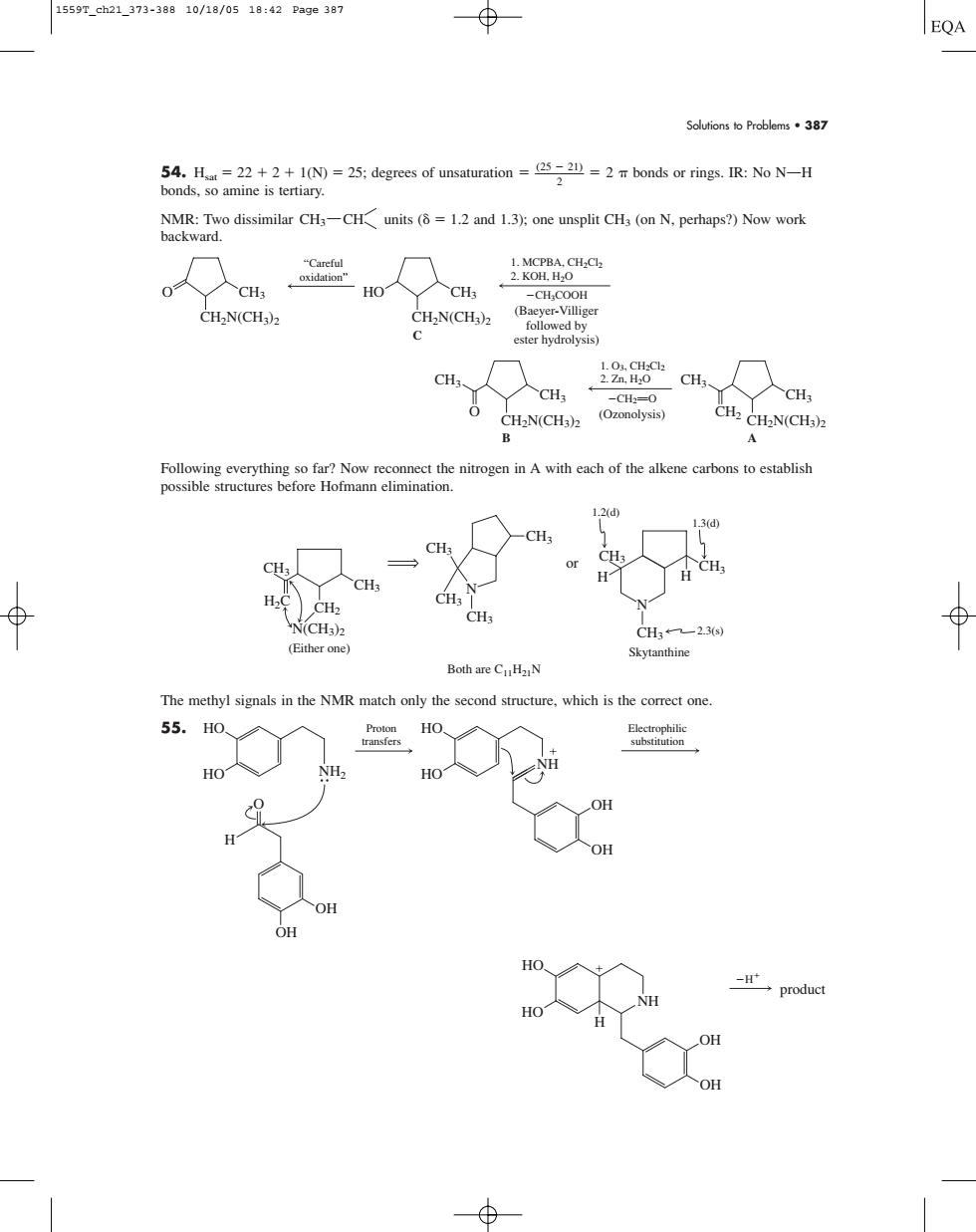
1559T_ch21_373-38810/18/0518:42Pa9e387 EQA Solutions to Problems.387 disimilar CH-CHuts (1and 1);one unsplit CH (on N.perhap?)Nowwork ondre CH: HO CH -CH-COOH CHN(CH CH-N(CHa)2 C a9 CH 人CH CH H CH2N(CHa)z 12d CH. CH. CH N(CH: CH-23) (Either one)】 Skytanthine Both are CuHz N The methyl signals in the NMR match only the second structure.which is the correct one 55. HO OH Solutions to Problems • 387 54. Hsat 22 2 1(N) 25; degrees of unsaturation
(25 2
21) 2
bonds or rings. IR: No NOH bonds, so amine is tertiary. NMR: Two dissimilar units ( 1.2 and 1.3); one unsplit CH3 (on N, perhaps?) Now work backward. Following everything so far? Now reconnect the nitrogen in A with each of the alkene carbons to establish possible structures before Hofmann elimination. The methyl signals in the NMR match only the second structure, which is the correct one. 55. H NH OH HO HO OH product H HO HO H OH O OH NH2 HO HO Proton transfers Electrophilic substitution NH OH OH N(CH3)2 (Either one) CH3 CH2 CH3 H2C CH3 CH3 CH3 CH3 N Skytanthine CH3 CH3 or H H CH3 N 1.2(d) 1.3(d) 2.3(s) Both are C11H21N CH2N(CH3)2 CH3 CH3 O 1. O3, CH2Cl2 2. Zn, H2O CH2PO (Ozonolysis) B CH2N(CH3)2 CH3 CH3 CH2 A “Careful oxidation” CH2N(CH3)2 O CH3 CH2N(CH3)2 HO CH3 C 1. MCPBA, CH2Cl2 2. KOH, H2O CH3COOH (Baeyer-Villiger followed by ester hydrolysis) CH3 CH 1559T_ch21_373-388 10/18/05 18:42 Page 387�����