正在加载图片...
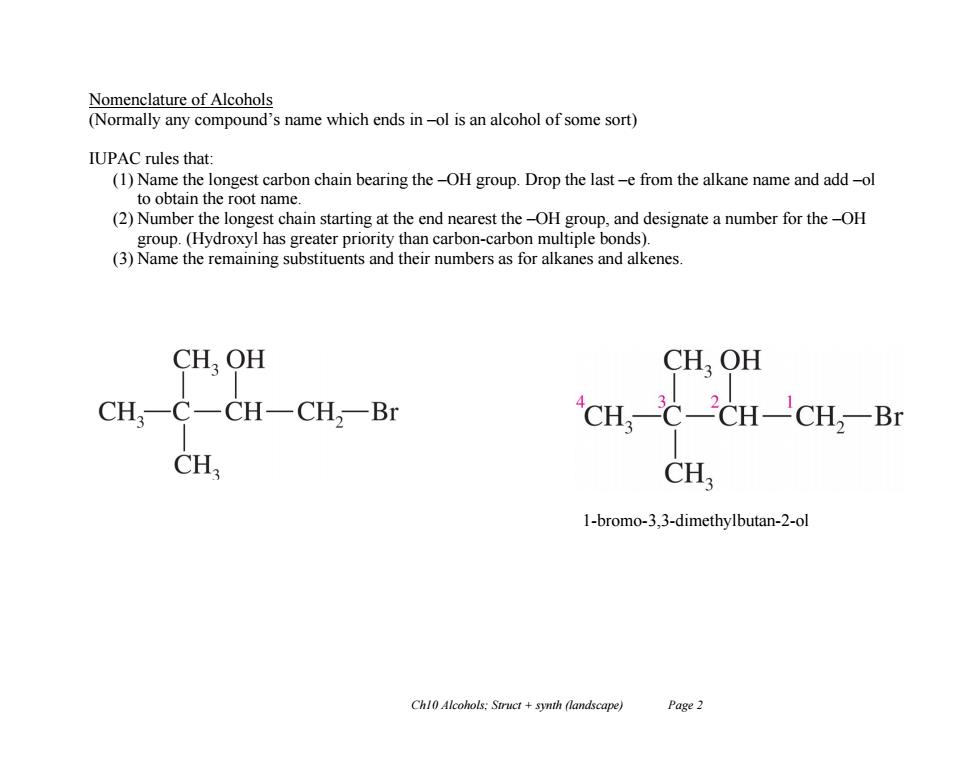
Nomenclature of Alcohols (Normally any compound's name which ends in-ol is an alcohol of some sort) IUPAC rules that: (1)Name the longest carbon chain bearing the-OH group.Drop the last-e from the alkane name and add-ol to obtain the root name. (2)Number the longest chain starting at the end nearest the-OH group,and designate a number for the-OH group.(Hydroxyl has greater priority than carbon-carbon multiple bonds). (3)Name the remaining substituents and their numbers as for alkanes and alkenes CH,OH CHOH CH,-C一CH-CH2-Br CHC-CH-CH:-Br CH CH 1-bromo-3,3-dimethylbutan-2-ol Chl0 Alcohols:Struct synth (landscape) Page 2Ch10 Alcohols; Struct + synth (landscape) Page 2 Nomenclature of Alcohols (Normally any compound’s name which ends in –ol is an alcohol of some sort) IUPAC rules that: (1) Name the longest carbon chain bearing the –OH group. Drop the last –e from the alkane name and add –ol to obtain the root name. (2) Number the longest chain starting at the end nearest the –OH group, and designate a number for the –OH group. (Hydroxyl has greater priority than carbon-carbon multiple bonds). (3) Name the remaining substituents and their numbers as for alkanes and alkenes. 1-bromo-3,3-dimethylbutan-2-ol