正在加载图片...
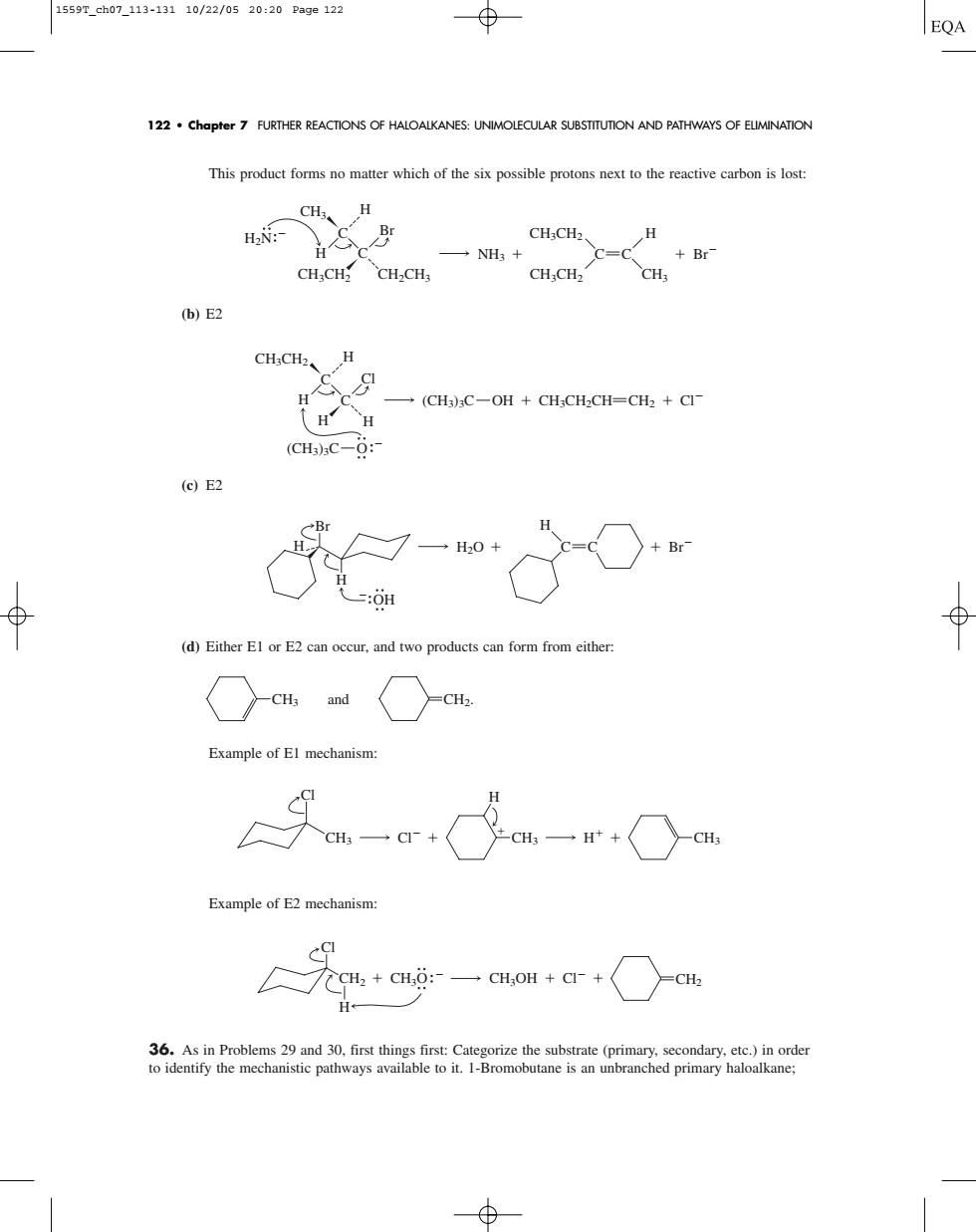
1559r.ah07.113-13110/22/0520:20Page122 122 chapter 7 FURTHER REACTONS OF HALOALKANES:UNIMOLECULAR SUBSTTTUTION AND PATHWAYS OF ELIMINATION This product forms no matter which of the six possible protons next to the reactive carbon is lost CH. CH:CH2 一NH+ +Br CH,CHCH.CH CH:CH2 CH (b)E2 CH,CH、 H -(CH3)C-OH CH;CH2CH=CH2 Cl (CH3)C-6: (c)E2 (d)Either EI or E2 can occur.and two products can form from either ○atw○at Example of El mechanism: Example of E2 mechanism a+a 一aot+cr+○aThis product forms no matter which of the six possible protons next to the reactive carbon is lost: (b) E2 (c) E2 (d) Either E1 or E2 can occur, and two products can form from either: Example of E1 mechanism: Example of E2 mechanism: 36. As in Problems 29 and 30, first things first: Categorize the substrate (primary, secondary, etc.) in order to identify the mechanistic pathways available to it. 1-Bromobutane is an unbranched primary haloalkane; CH2 H CH3 CH3OH Cl Cl O CH2 CH3 CH3 H CH3 Cl Cl H CH3 and CH2. H2O H Br OH H C C H Br OH CH3CH2CH H CH3CH2 (CH3)3C CH2 O Cl (CH3)3C C C Cl H H H C CH3CH2 CH3CH2 CH2CH3 CH3CH2 CH3 H CH3 C Br C NH3 C Br H H H2N 122 • Chapter 7 FURTHER REACTIONS OF HALOALKANES: UNIMOLECULAR SUBSTITUTION AND PATHWAYS OF ELIMINATION 1559T_ch07_113-131 10/22/05 20:20 Page 122�������������