正在加载图片...
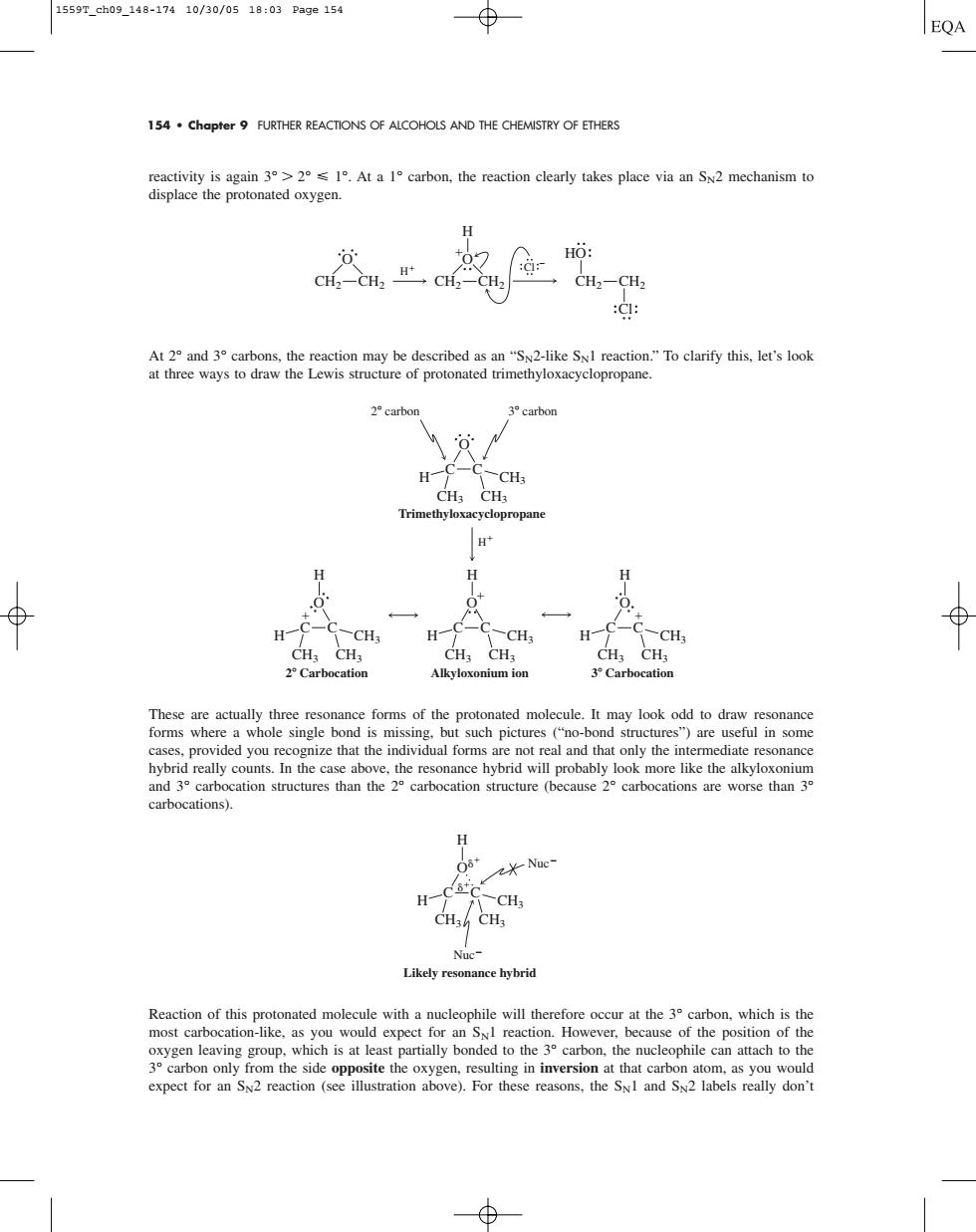
1559Tch09148-17410/30/0518:03Page154 154.chapter9 FURTHER REACTIONS OF ALCOHOLS AND THE CHEMISTRY OF ETHERS reactivity is again31.At a 1 carbon,the reaction clearly takes place via an Sx2 mechanism to displace the protonated oxygen. -CH2-CH2 At 2 and 3 carbons,the reaction may be described as an "SN2-like SyI reaction."To clarify this,let's look at three ways to draw the Lewis structure of protonated trimethyloxacyclopropane. carbo H CH: a- H LC-0 H CH, H CH; H C ated molecule.It may look odd to e resonanc carbocations). H H- Nuc -CH; Likely resonance hybrid Reaction of this protonated molecule with a nucleophile will therefore occur at the 3 carbon,which is the beca oxygccubocation-like hch cast partially expect for an S2 reaction (see illustration above).For these reasons.the Sv1 and S2 labels really don't154 • Chapter 9 FURTHER REACTIONS OF ALCOHOLS AND THE CHEMISTRY OF ETHERS reactivity is again 3° 2° 1°. At a 1° carbon, the reaction clearly takes place via an SN2 mechanism to displace the protonated oxygen. At 2° and 3° carbons, the reaction may be described as an “SN2-like SN1 reaction.” To clarify this, let’s look at three ways to draw the Lewis structure of protonated trimethyloxacyclopropane. These are actually three resonance forms of the protonated molecule. It may look odd to draw resonance forms where a whole single bond is missing, but such pictures (“no-bond structures”) are useful in some cases, provided you recognize that the individual forms are not real and that only the intermediate resonance hybrid really counts. In the case above, the resonance hybrid will probably look more like the alkyloxonium and 3° carbocation structures than the 2° carbocation structure (because 2° carbocations are worse than 3° carbocations). Reaction of this protonated molecule with a nucleophile will therefore occur at the 3° carbon, which is the most carbocation-like, as you would expect for an SN1 reaction. However, because of the position of the oxygen leaving group, which is at least partially bonded to the 3° carbon, the nucleophile can attach to the 3° carbon only from the side opposite the oxygen, resulting in inversion at that carbon atom, as you would expect for an SN2 reaction (see illustration above). For these reasons, the SN1 and SN2 labels really don’t H O H C C CH3 CH3 CH3 Likely resonance hybrid Nuc Nuc H H C C O CH3 CH3 CH3 O H Trimethyloxacyclopropane H C C CH3 CH3 CH3 Alkyloxonium ion H O H C C CH3 CH3 CH3 3 Carbocation H O H C C CH3 CH3 CH3 2 Carbocation 2 carbon 3 carbon CH2 CH2 HO H O CH2 CH2 O H CH2 CH2 Cl Cl 1559T_ch09_148-174 10/30/05 18:03 Page 154������������