正在加载图片...
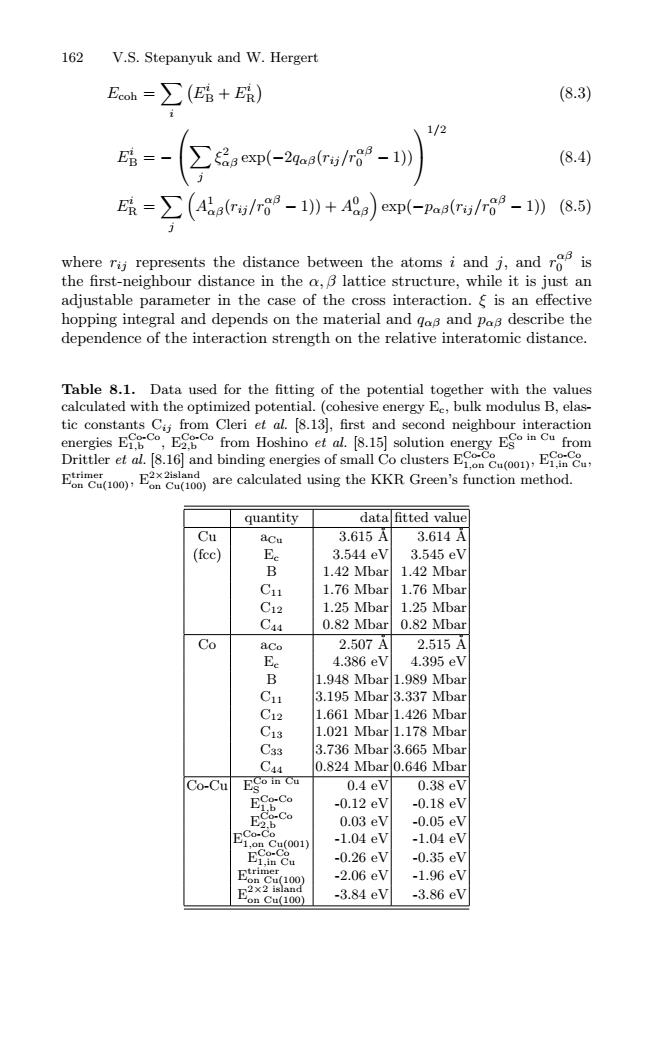
162 V.S.Stepanyuk and W.Hergert Eoh=∑(E路+R) (8.3) 1/2 -2aa/-l (8.4) E=∑((Aier/r88-1)+4A8a)ep(-pas(r/n6-10 (8.5) whererij represents the distance between the atoms i and j,and ro is the first-neighbour distance in the a,B lattice structure,while it is just an adjustable parameter in the case of the cross interaction.is an effective hopping integral and depends on the material and gos and pas describe the dependence of the interaction strength on the relative interatomic distance. Table 8.1.Data used for the fitting of the potential together with the values calculated with the optimized potential.(cohesive energy Ec,bulk modulus B,elas- tic constants C from Cleri et al.[8.13],first and second neighbour interaction energies Efgc,Eco from Hoshino et al.8.15]solution energy Eon Cu from Drittler etal 816]and binding energies of small CoclustersE)E E2x2island oncu(oo)are calculated using the KKR Green's function method. quantity data fitted value Cu aCu 3.615A 3.614A (fcc) e 3.544eV 3.545eV 1.42 Mbar 1.42 Mbar C11 1.76 Mbar 1.76 Mbar C12 1.25 Mbar 1.25 Mbar C44 0.82 Mbar 0.82 Mbar Co aC 2.507A 2.515A Ee 4.386eV 4.395eV B 1.948 Mbar 1.989 Mbar C1 3.195 Mbar3.337 Mbar C12 1.661 Mbar 1.426 Mbar C13 1.021 Mbar 1.178 Mbar C33 3.736 Mbar 3.665 Mbar C44 0.824 Mbar 0.646 Mbar ●o-Cu Eso in Cu 0.4eV 0.38eV -0.12eV -0.18eV 0.03eV -0.05eV ECu(001) -1.04eV -1.04eV ECn Cu -0.26eV -0.35eV -2.06eV -1.96eV -3.84eV -3.86eV162 V.S. Stepanyuk and W. Hergert Ecoh = i Ei B + Ei R (8.3) Ei B = − j ξ2 αβ exp(−2qαβ(rij/rαβ 0 − 1)) 1/2 (8.4) Ei R = j A1 αβ(rij/rαβ 0 − 1)) + A0 αβ exp(−pαβ(rij/rαβ 0 − 1)) (8.5) where rij represents the distance between the atoms i and j, and r αβ 0 is the first-neighbour distance in the α, β lattice structure, while it is just an adjustable parameter in the case of the cross interaction. ξ is an effective hopping integral and depends on the material and qαβ and pαβ describe the dependence of the interaction strength on the relative interatomic distance. Table 8.1. Data used for the fitting of the potential together with the values calculated with the optimized potential. (cohesive energy Ec, bulk modulus B, elastic constants Cij from Cleri et al. [8.13], first and second neighbour interaction energies ECo-Co 1,b , ECo-Co 2,b from Hoshino et al. [8.15] solution energy ECo in Cu S from Drittler et al. [8.16] and binding energies of small Co clusters ECo-Co 1,on Cu(001), ECo-Co 1,in Cu, Etrimer on Cu(100), E2×2island on Cu(100) are calculated using the KKR Green’s function method. quantity data fitted value Cu aCu 3.615 ˚A 3.614 ˚A (fcc) Ec 3.544 eV 3.545 eV B 1.42 Mbar 1.42 Mbar C11 1.76 Mbar 1.76 Mbar C12 1.25 Mbar 1.25 Mbar C44 0.82 Mbar 0.82 Mbar Co aCo 2.507 ˚A 2.515 ˚A Ec 4.386 eV 4.395 eV B 1.948 Mbar 1.989 Mbar C11 3.195 Mbar 3.337 Mbar C12 1.661 Mbar 1.426 Mbar C13 1.021 Mbar 1.178 Mbar C33 3.736 Mbar 3.665 Mbar C44 0.824 Mbar 0.646 Mbar Co-Cu ECo in Cu S 0.4 eV 0.38 eV ECo-Co 1,b -0.12 eV -0.18 eV ECo-Co 2,b 0.03 eV -0.05 eV ECo-Co 1,on Cu(001) -1.04 eV -1.04 eV ECo-Co 1,in Cu -0.26 eV -0.35 eV Etrimer on Cu(100) -2.06 eV -1.96 eV E2×2 island on Cu(100) -3.84 eV -3.86 eV