正在加载图片...
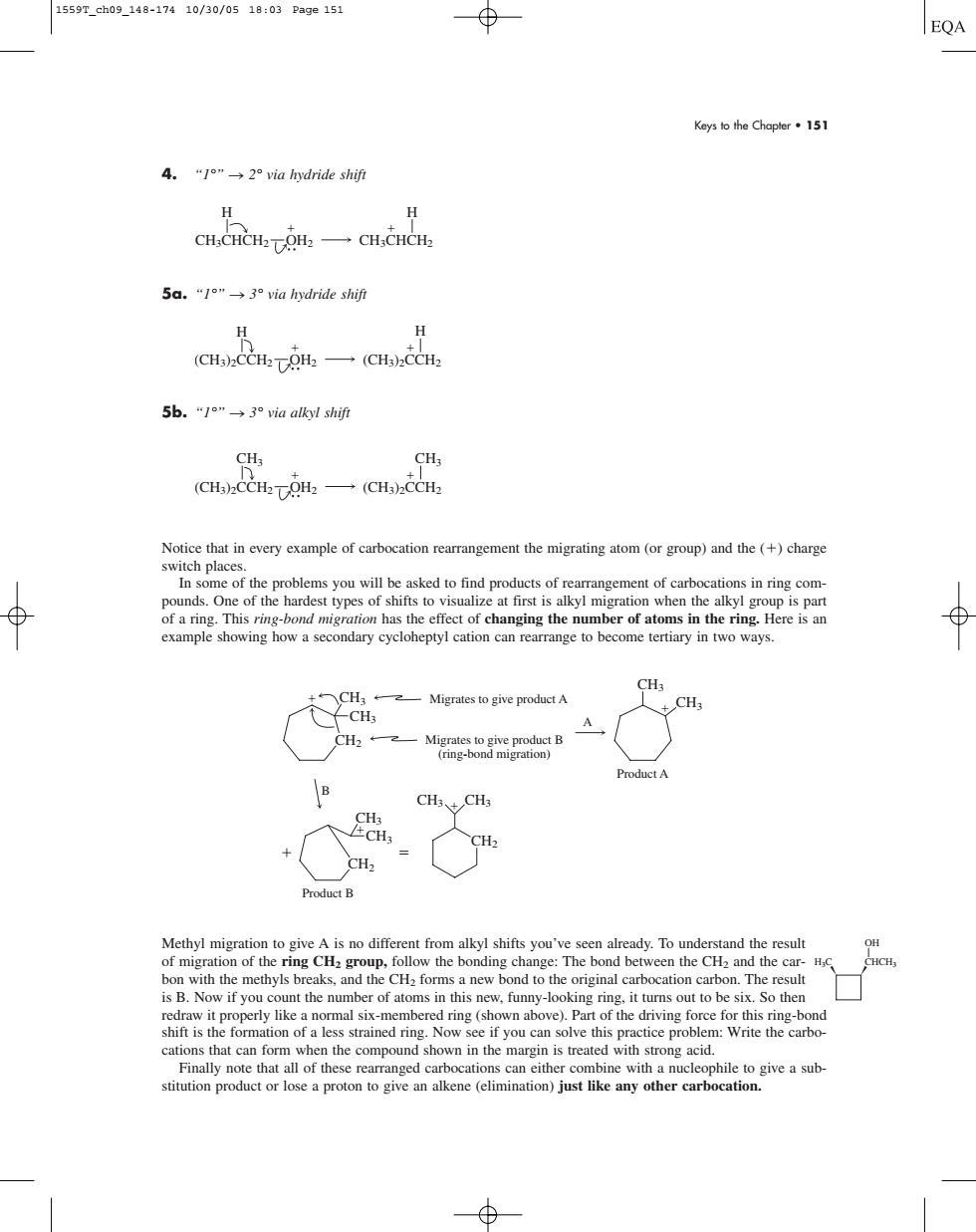
1559T_ch09_148-17410/30/0518:03Pa9e151 EQA Keys to the Chapler 151 4."1"→2°via hydride shif H CH,CHCH-TOH一CH,HC 5a.“/o"→3 via hydride shift (CH)CCH2TOH2(CHa)CCH2 5b.“1"→3°via alkyl shif0 ana一an Notice that in every example of carbocation rearrangement the migrating atom (or group)and the(+)charge switch places of the of changing the number of atoms in theing Heres example showing how a secondary cycloheptyl cation can rearrange to become tertiary in two ways. CH CH Migrates to give productA 一Min用 Product A B Product B Methyl migration to give A is no different from alkyl shifts you've seen already.To understand the result The bond between the CHa and the ca is B.Now if you count the number of atoms in this new.funny-lookinrin it turns out to be six So then cations that can form when the compound shown in the marin is reated ng acid Keys to the Chapter • 151 4. “1°” n 2° via hydride shift 5a. “1°” n 3° via hydride shift 5b. “1°” n 3° via alkyl shift Notice that in every example of carbocation rearrangement the migrating atom (or group) and the () charge switch places. In some of the problems you will be asked to find products of rearrangement of carbocations in ring compounds. One of the hardest types of shifts to visualize at first is alkyl migration when the alkyl group is part of a ring. This ring-bond migration has the effect of changing the number of atoms in the ring. Here is an example showing how a secondary cycloheptyl cation can rearrange to become tertiary in two ways. Methyl migration to give A is no different from alkyl shifts you’ve seen already. To understand the result of migration of the ring CH2 group, follow the bonding change: The bond between the CH2 and the carbon with the methyls breaks, and the CH2 forms a new bond to the original carbocation carbon. The result is B. Now if you count the number of atoms in this new, funny-looking ring, it turns out to be six. So then redraw it properly like a normal six-membered ring (shown above). Part of the driving force for this ring-bond shift is the formation of a less strained ring. Now see if you can solve this practice problem: Write the carbocations that can form when the compound shown in the margin is treated with strong acid. Finally note that all of these rearranged carbocations can either combine with a nucleophile to give a substitution product or lose a proton to give an alkene (elimination) just like any other carbocation. CH2 CH3 CH3 CH3 CH3 CH2 CH3 CH3 Migrates to give product A Migrates to give product B (ring-bond migration) Product A A CH2 CH3 CH3 Product B B (CH3)2CCH2 CH3 (CH3)2CCH2 CH3 OH2 (CH3)2CCH2 OH2 H (CH3)2CCH2 H CH3CHCH2 H CH3CHCH2 H OH2 H3C CHCH3 OH 1559T_ch09_148-174 10/30/05 18:03 Page 151������������