正在加载图片...
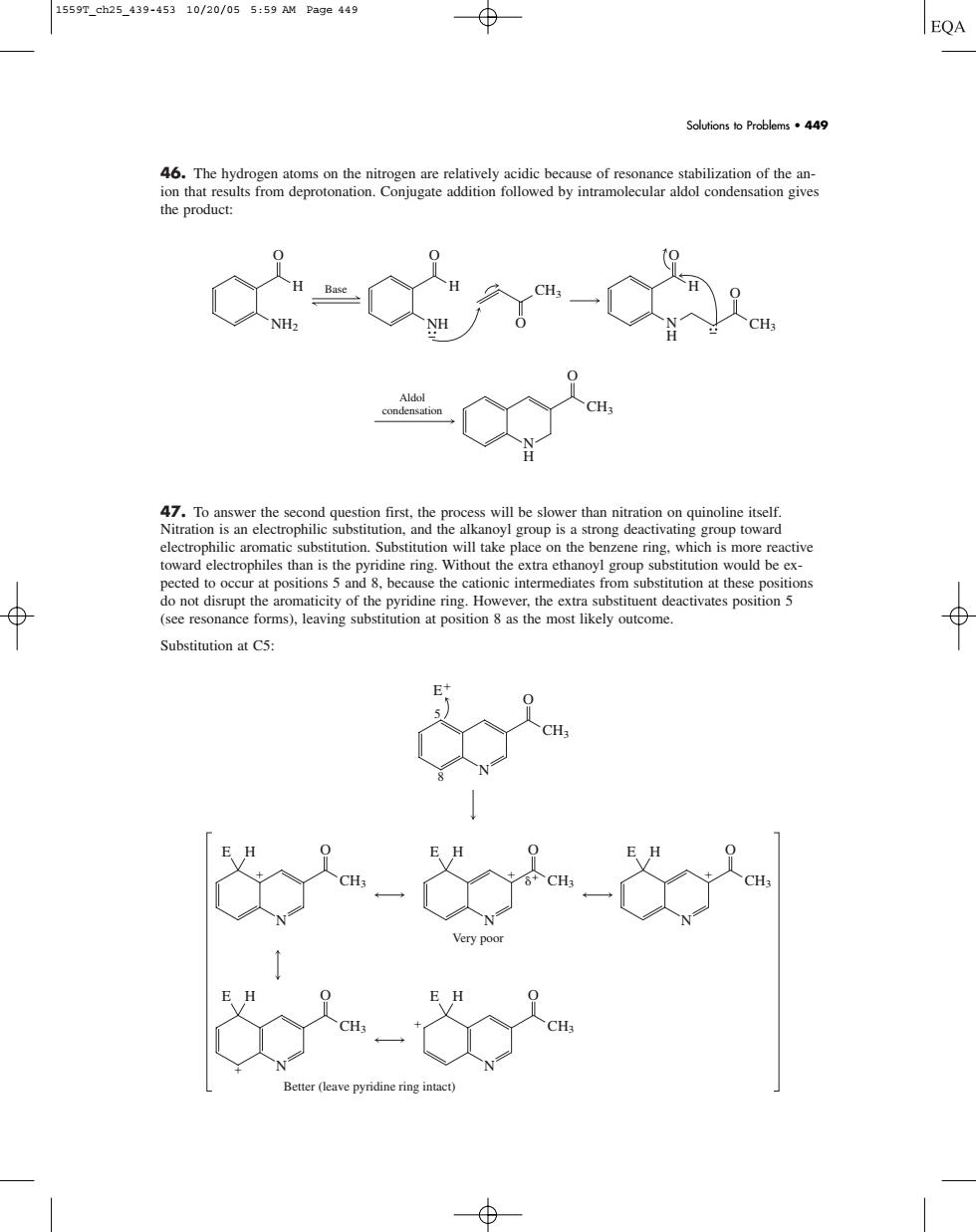
1559T_ch25439-45310/20/055:59 AM Page44g ⊕ EQA Solutions to Problems.449 the product: -0 aromatic substitution.take place on the benzene ring.which is more reactive toward electrophiles than is the pyridine ring.Without the extra ethanoyl group substitution would be ex- te ca of th (see resonance forms),leaving substitution at position 8 as the most likely outcome. Substitution at C5 46. The hydrogen atoms on the nitrogen are relatively acidic because of resonance stabilization of the anion that results from deprotonation. Conjugate addition followed by intramolecular aldol condensation gives the product: 47. To answer the second question first, the process will be slower than nitration on quinoline itself. Nitration is an electrophilic substitution, and the alkanoyl group is a strong deactivating group toward electrophilic aromatic substitution. Substitution will take place on the benzene ring, which is more reactive toward electrophiles than is the pyridine ring. Without the extra ethanoyl group substitution would be expected to occur at positions 5 and 8, because the cationic intermediates from substitution at these positions do not disrupt the aromaticity of the pyridine ring. However, the extra substituent deactivates position 5 (see resonance forms), leaving substitution at position 8 as the most likely outcome. Substitution at C5: O CH3 N
E H Very poor O CH3 N O E H CH3 N E H O CH3 N O E H CH3 N E H Better (leave pyridine ring intact) O CH3 8 5 N E Aldol condensation O CH3 N H Base O NH2 H O NH H O H O CH3 O CH3 N H Solutions to Problems • 449 1559T_ch25_439-453 10/20/05 5:59 AM Page 449