正在加载图片...
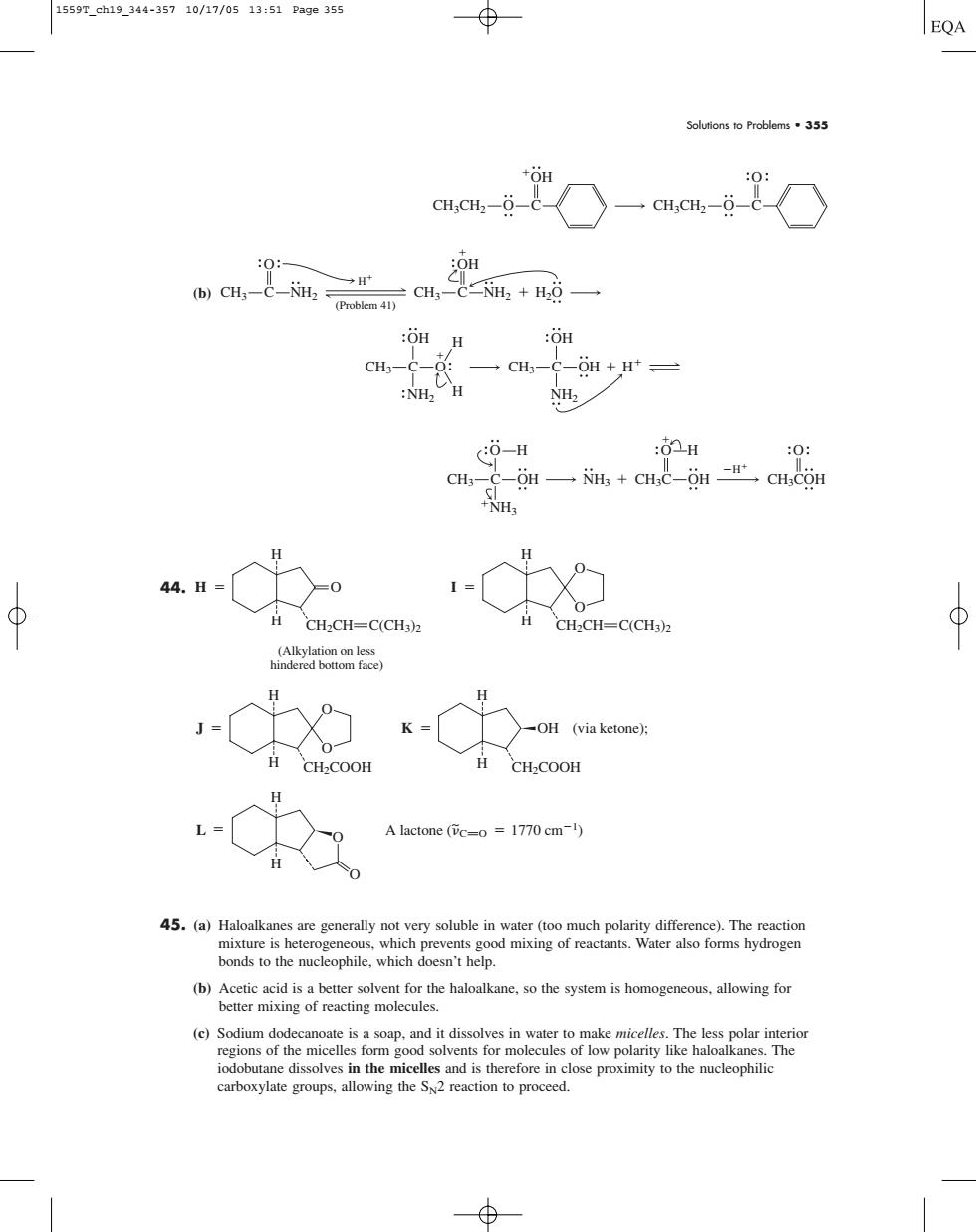
1559T_ch19_344-35710/17/0513:51Pa9e355 EQA Soufions to Problems55 0 :OH :OH H cH-C-:一cH,-C-n+,H* 0-H :0H 0: CH--OH-NH +CHC-SH CH.COH +NH 44H-0 日cH,CH=cCH2 CH:CH=C(CH3)2 (on -c -OH (via ketone CH-COOH CH2COOH A lactone (Tc-o=1770 cm-1) bonds to the nucleophile.which doesn't help. Solutions to Problems • 355 (b) 44. 45. (a) Haloalkanes are generally not very soluble in water (too much polarity difference). The reaction mixture is heterogeneous, which prevents good mixing of reactants. Water also forms hydrogen bonds to the nucleophile, which doesn’t help. (b) Acetic acid is a better solvent for the haloalkane, so the system is homogeneous, allowing for better mixing of reacting molecules. (c) Sodium dodecanoate is a soap, and it dissolves in water to make micelles. The less polar interior regions of the micelles form good solvents for molecules of low polarity like haloalkanes. The iodobutane dissolves in the micelles and is therefore in close proximity to the nucleophilic carboxylate groups, allowing the SN2 reaction to proceed. L A lactone ( C H H O O O 1770 cm1) ~ CH2COOH J O O H H CH2COOH K OH (via ketone); H H CH2CH C(CH3)2 H O H H (Alkylation on less hindered bottom face) CH2CH C(CH3)2 I O O H H O CH3 C OH NH3 NH3 H O CH3C OH H O CH3COH H CH3 C O NH2 OH H H CH3 C OH NH2 OH H O CH3 C NH2 CH3 C NH2 OH H (Problem 41) H2O CH3CH2 O C OH CH3CH2 O C O 1559T_ch19_344-357 10/17/05 13:51 Page 355�����������