正在加载图片...
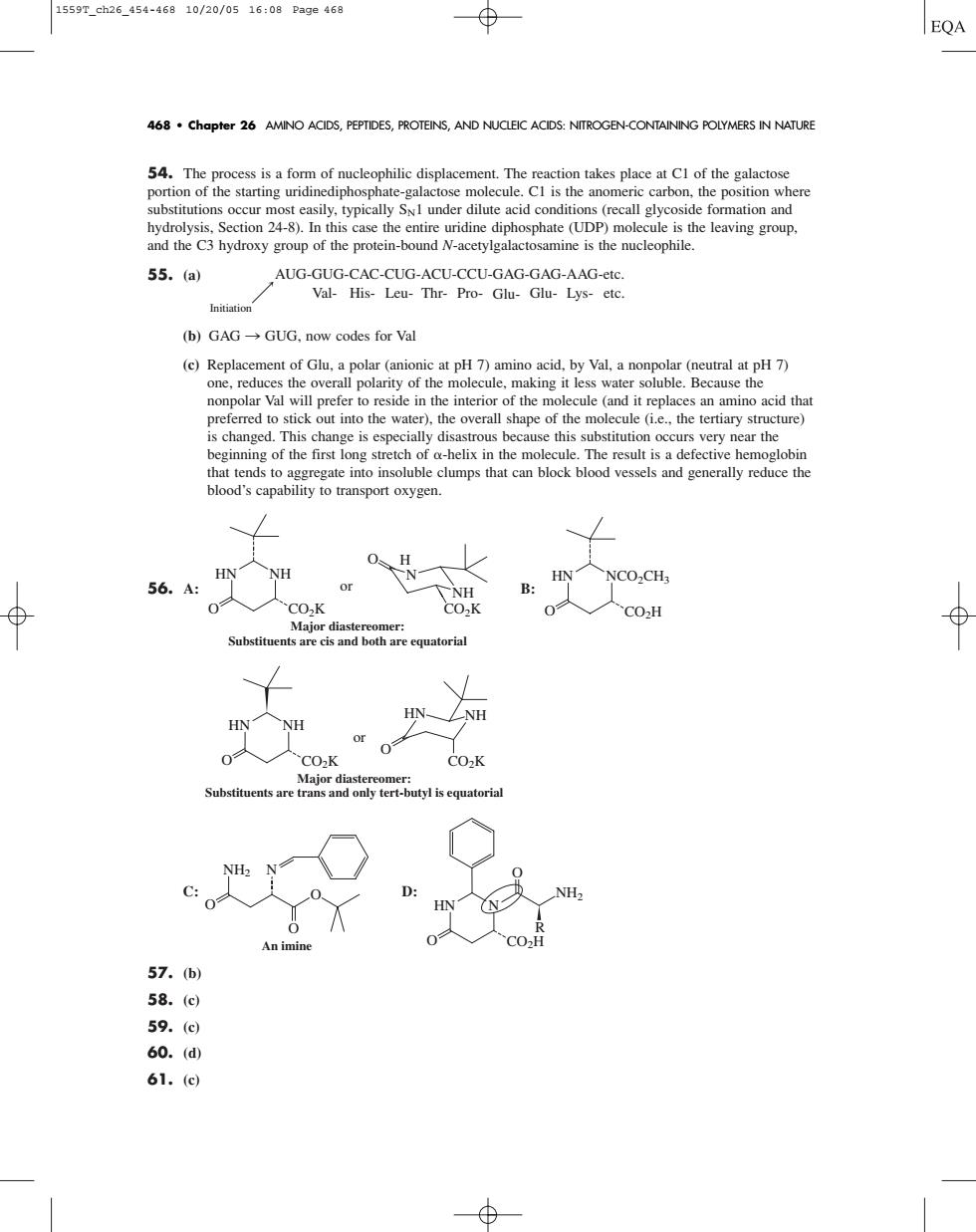
15597.ah26.454-46810/20/0516:09Page468 EQA 468 chapter 26 AMINO ACIDS,PEPTIDES,PROTEINS,AND NUCLEI ACIDS:NITROGEN-CONTAINING POLYMERS IN NATURE most sily acid conditions (formation and hydrolysis.Section 24-8).In this case the entire uridine diphosphate (UDP)molecule is the leaving group. and the C3 hydroxy group of the protein-bound N-acetylgalactosamine is the nucleophile. 55.a AUG-GUG-CAC-CUG-ACU-CCU-GAG-GAG-AAG-etc. Val-His-Leu-Thr-Pro-Glu-Glu-Lys-etc. (b)GAG-GUG,now codes for Val (c)Replacement of Glu,apolar (anionic at pH)amino acid.by Val,a nonpolar (neutral at pH7) one.e overall polant y0n e.Because th is changed.This change is especially disastrous because this substitution occurs very near the / H NH 56. A HNNCO,CH B: CO-K r di and both re equa Suber-utyqu 57.b) 58. 59.(d) 60.(d 61.c54. The process is a form of nucleophilic displacement. The reaction takes place at C1 of the galactose portion of the starting uridinediphosphate-galactose molecule. C1 is the anomeric carbon, the position where substitutions occur most easily, typically SN1 under dilute acid conditions (recall glycoside formation and hydrolysis, Section 24-8). In this case the entire uridine diphosphate (UDP) molecule is the leaving group, and the C3 hydroxy group of the protein-bound N-acetylgalactosamine is the nucleophile. 55. (a) (b) GAG n GUG, now codes for Val (c) Replacement of Glu, a polar (anionic at pH 7) amino acid, by Val, a nonpolar (neutral at pH 7) one, reduces the overall polarity of the molecule, making it less water soluble. Because the nonpolar Val will prefer to reside in the interior of the molecule (and it replaces an amino acid that preferred to stick out into the water), the overall shape of the molecule (i.e., the tertiary structure) is changed. This change is especially disastrous because this substitution occurs very near the beginning of the first long stretch of -helix in the molecule. The result is a defective hemoglobin that tends to aggregate into insoluble clumps that can block blood vessels and generally reduce the blood’s capability to transport oxygen. 56. A: B: C: D: 57. (b) 58. (c) 59. (c) 60. (d) 61. (c) HN N R O O CO2H NH2 NH2 N O O O An imine HN NH O O CO2K CO2K or Major diastereomer: Substituents are trans and only tert-butyl is equatorial HN NH HN NCO2CH3 O CO2H HN NH O O CO2K CO2K or H N NH Major diastereomer: Substituents are cis and both are equatorial AUG-GUG-CAC-CUG-ACU-CCU-GAG-GAG-AAG-etc. Val- His- Leu- Thr- Pro- Glu- Glu- Lys- etc. Initiation 468 • Chapter 26 AMINO ACIDS, PEPTIDES, PROTEINS, AND NUCLEIC ACIDS: NITROGEN-CONTAINING POLYMERS IN NATURE 1559T_ch26_454-468 10/20/05 16:08 Page 468