正在加载图片...
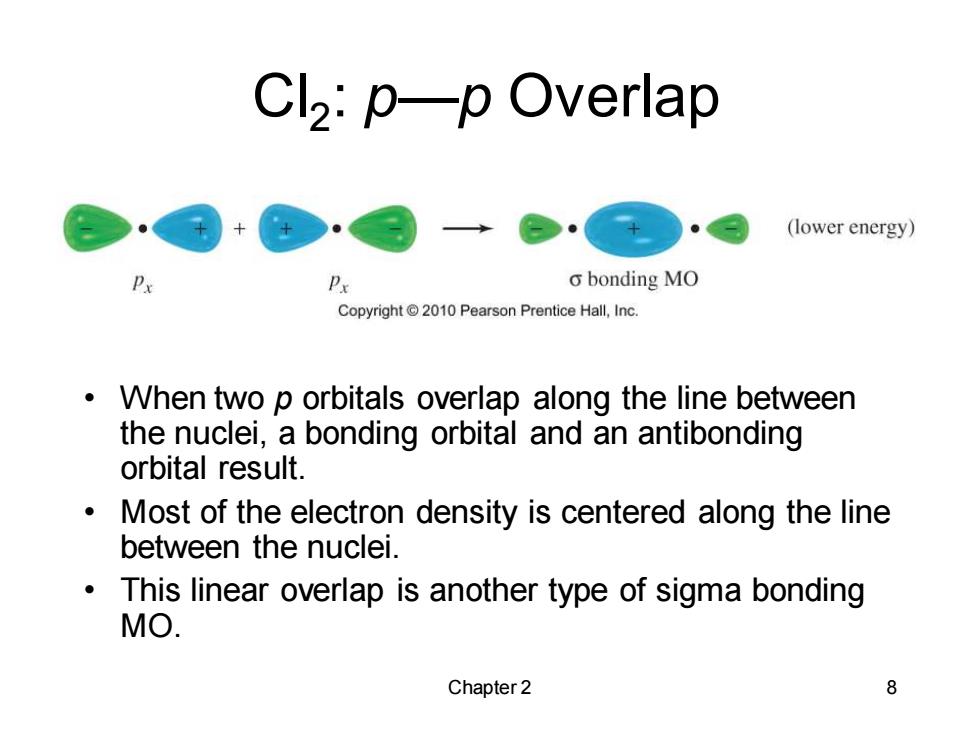
Cl2:p-p Overlap (lower energy) P Px o bonding MO Copyright 2010 Pearson Prentice Hall,Inc. When two p orbitals overlap along the line between the nuclei,a bonding orbital and an antibonding orbital result. Most of the electron density is centered along the line between the nuclei. This linear overlap is another type of sigma bonding MO. Chapter 2 8 Chapter 2 8 Cl2 : p—p Overlap • When two p orbitals overlap along the line between the nuclei, a bonding orbital and an antibonding orbital result. • Most of the electron density is centered along the line between the nuclei. • This linear overlap is another type of sigma bonding MO