正在加载图片...
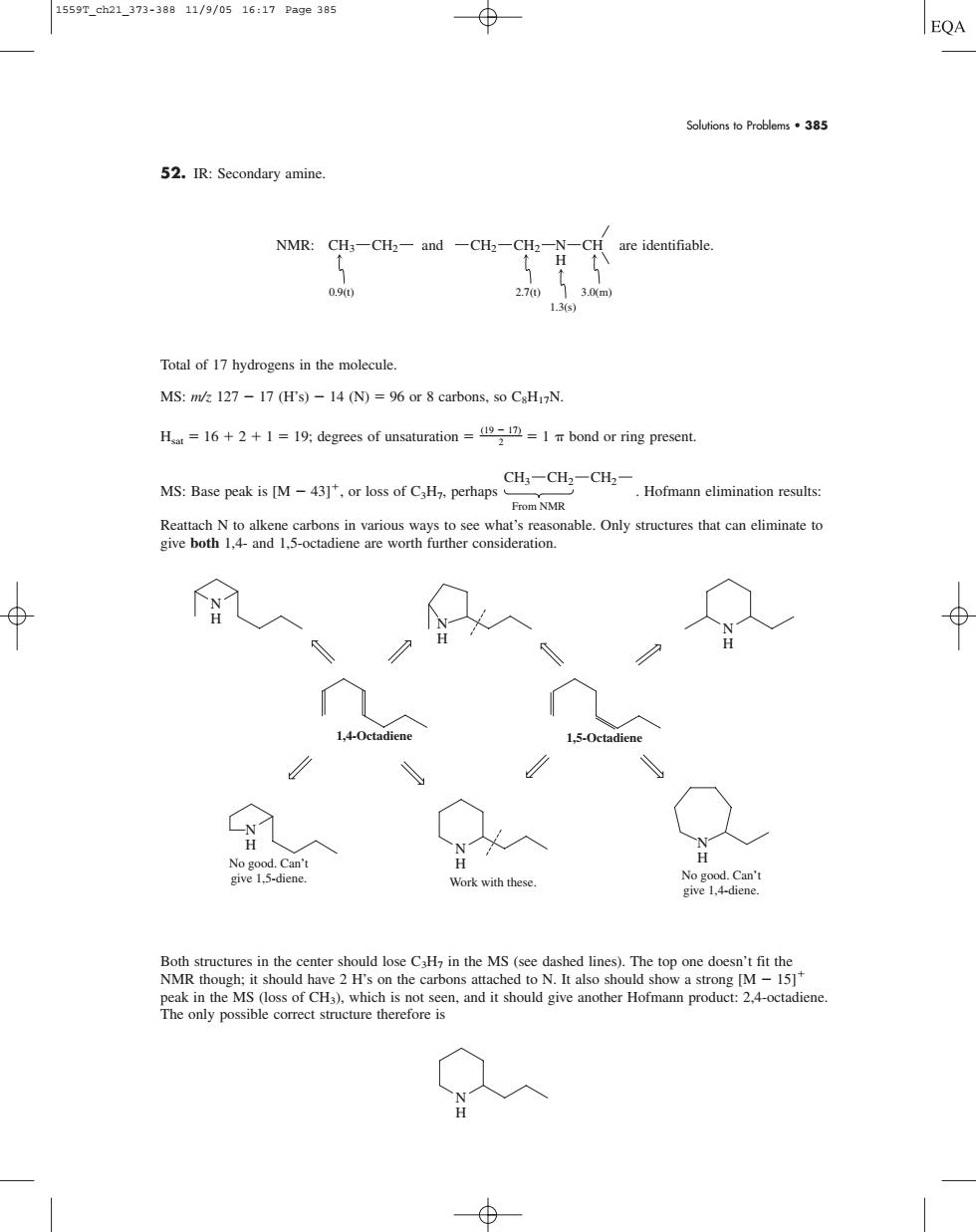
1559T_ch21_373-38811/9/0516:17Pae385 EQA Solutions to Problems.385 52.IR:Secondary amine NMR:CH-CH-and-CH2-CH2-N -CH re identifiable. 2.70 1.3 Total of 17 hydrogens in the molecule. MS:m/127-17 (H's)-14 (N)=96 or 8 carbons,so CsHzN. H=16++119 degrees of unsaturation=1 bond or ring present. MS:Base pk is (Mo CHCCH.- Hofmann elimination results: From NMR Reattach N to alkene carbons in various ways to see what's reasonable.Only structures that can eliminate to give both 1.4-and 1.5-octadicne are worth further consideration. L4.0c N Work with these. eak in th MS (osofCH)which isnotsen and it shoudive ther Homa product:. The only possible correct structure therefore is Solutions to Problems • 385 52. IR: Secondary amine. are identifiable. Total of 17 hydrogens in the molecule. MS: m/z 127 17 (H’s) 14 (N) 96 or 8 carbons, so C8H17N. Hsat 16 2 1 19; degrees of unsaturation (19 2 17) 1 bond or ring present. MS: Base peak is [M 43], or loss of C3H7, perhaps . Hofmann elimination results: Reattach N to alkene carbons in various ways to see what’s reasonable. Only structures that can eliminate to give both 1,4- and 1,5-octadiene are worth further consideration. Both structures in the center should lose C3H7 in the MS (see dashed lines). The top one doesn’t fit the NMR though; it should have 2 H’s on the carbons attached to N. It also should show a strong [M 15] peak in the MS (loss of CH3), which is not seen, and it should give another Hofmann product: 2,4-octadiene. The only possible correct structure therefore is N H N H N H N H N H N H N H 1,4-Octadiene 1,5-Octadiene Work with these. No good. Can’t give 1,5-diene. No good. Can’t give 1,4-diene. CH3 CH2 CH2 From NMR N CH H NMR: and CH3 CH2 CH2 CH2 0.9(t) 2.7(t) 3.0(m) 1.3(s) 1559T_ch21_373-388 11/9/05 16:17 Page 385����������