正在加载图片...
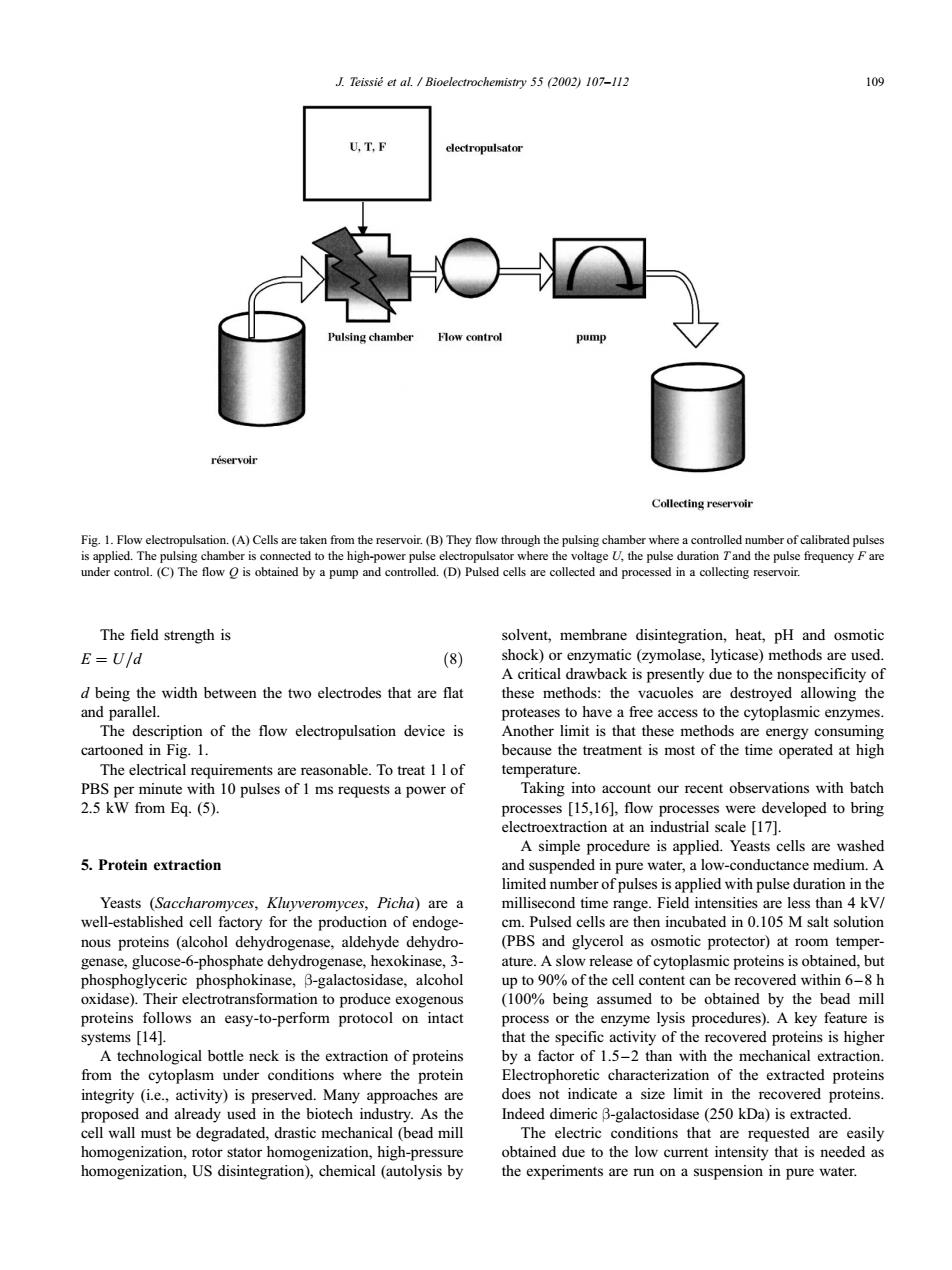
J.Teissie et al.Bioelectrochemistry 55 (2002)107-112 109 U,T,F electropulsator Pulsing chamber Flow control pump reservoir Collecting reservoir Fig.1.Flow electropulsation.(A)Cells are taken from the reservoir.(B)They flow through the pulsing chamber where a controlled number of calibrated pulses is applied.The pulsing chamber is connected to the high-power pulse electropulsator where the voltage U,the pulse duration Tand the pulse frequency F are under control.(C)The flow o is obtained by a pump and controlled.(D)Pulsed cells are collected and processed in a collecting reservoir. The field strength is solvent,membrane disintegration,heat,pH and osmotic E=U/d (8) shock)or enzymatic (zymolase,lyticase)methods are used. A critical drawback is presently due to the nonspecificity of d being the width between the two electrodes that are flat these methods:the vacuoles are destroyed allowing the and parallel. proteases to have a free access to the cytoplasmic enzymes. The description of the flow electropulsation device is Another limit is that these methods are energy consuming cartooned in Fig.1. because the treatment is most of the time operated at high The electrical requirements are reasonable.To treat 1 I of temperature. PBS per minute with 10 pulses of 1 ms requests a power of Taking into account our recent observations with batch 2.5 kW from Eq.(5). processes [15,16],flow processes were developed to bring electroextraction at an industrial scale [17]. A simple procedure is applied.Yeasts cells are washed 5.Protein extraction and suspended in pure water,a low-conductance medium.A limited number of pulses is applied with pulse duration in the Yeasts (Saccharomyces,Kluyveromyces,Picha)are a millisecond time range.Field intensities are less than 4 kV/ well-established cell factory for the production of endoge- cm.Pulsed cells are then incubated in 0.105 M salt solution nous proteins (alcohol dehydrogenase,aldehyde dehydro- (PBS and glycerol as osmotic protector)at room temper- genase,glucose-6-phosphate dehydrogenase,hexokinase,3- ature.A slow release of cytoplasmic proteins is obtained,but phosphoglyceric phosphokinase,B-galactosidase,alcohol up to 90%of the cell content can be recovered within 6-8 h oxidase).Their electrotransformation to produce exogenous (100%being assumed to be obtained by the bead mill proteins follows an easy-to-perform protocol on intact process or the enzyme lysis procedures).A key feature is systems [14]. that the specific activity of the recovered proteins is higher A technological bottle neck is the extraction of proteins by a factor of 1.5-2 than with the mechanical extraction. from the cytoplasm under conditions where the protein Electrophoretic characterization of the extracted proteins integrity (i.e.,activity)is preserved.Many approaches are does not indicate a size limit in the recovered proteins. proposed and already used in the biotech industry.As the Indeed dimeric B-galactosidase(250 kDa)is extracted. cell wall must be degradated,drastic mechanical (bead mill The electric conditions that are requested are easily homogenization,rotor stator homogenization,high-pressure obtained due to the low current intensity that is needed as homogenization,US disintegration),chemical(autolysis by the experiments are run on a suspension in pure water.The field strength is E ¼ U=d ð8Þ d being the width between the two electrodes that are flat and parallel. The description of the flow electropulsation device is cartooned in Fig. 1. The electrical requirements are reasonable. To treat 1 l of PBS per minute with 10 pulses of 1 ms requests a power of 2.5 kW from Eq. (5). 5. Protein extraction Yeasts (Saccharomyces, Kluyveromyces, Picha) are a well-established cell factory for the production of endogenous proteins (alcohol dehydrogenase, aldehyde dehydrogenase, glucose-6-phosphate dehydrogenase, hexokinase, 3- phosphoglyceric phosphokinase, b-galactosidase, alcohol oxidase). Their electrotransformation to produce exogenous proteins follows an easy-to-perform protocol on intact systems [14]. A technological bottle neck is the extraction of proteins from the cytoplasm under conditions where the protein integrity (i.e., activity) is preserved. Many approaches are proposed and already used in the biotech industry. As the cell wall must be degradated, drastic mechanical (bead mill homogenization, rotor stator homogenization, high-pressure homogenization, US disintegration), chemical (autolysis by solvent, membrane disintegration, heat, pH and osmotic shock) or enzymatic (zymolase, lyticase) methods are used. A critical drawback is presently due to the nonspecificity of these methods: the vacuoles are destroyed allowing the proteases to have a free access to the cytoplasmic enzymes. Another limit is that these methods are energy consuming because the treatment is most of the time operated at high temperature. Taking into account our recent observations with batch processes [15,16], flow processes were developed to bring electroextraction at an industrial scale [17]. A simple procedure is applied. Yeasts cells are washed and suspended in pure water, a low-conductance medium. A limited number of pulses is applied with pulse duration in the millisecond time range. Field intensities are less than 4 kV/ cm. Pulsed cells are then incubated in 0.105 M salt solution (PBS and glycerol as osmotic protector) at room temperature. A slow release of cytoplasmic proteins is obtained, but up to 90% of the cell content can be recovered within 6 –8 h (100% being assumed to be obtained by the bead mill process or the enzyme lysis procedures). A key feature is that the specific activity of the recovered proteins is higher by a factor of 1.5 –2 than with the mechanical extraction. Electrophoretic characterization of the extracted proteins does not indicate a size limit in the recovered proteins. Indeed dimeric b-galactosidase (250 kDa) is extracted. The electric conditions that are requested are easily obtained due to the low current intensity that is needed as the experiments are run on a suspension in pure water. Fig. 1. Flow electropulsation. (A) Cells are taken from the reservoir. (B) They flow through the pulsing chamber where a controlled number of calibrated pulses is applied. The pulsing chamber is connected to the high-power pulse electropulsator where the voltage U, the pulse duration T and the pulse frequency F are under control. (C) The flow Q is obtained by a pump and controlled. (D) Pulsed cells are collected and processed in a collecting reservoir. J. Teissie´ et al. / Bioelectrochemistry 55 (2002) 107–112 109