正在加载图片...
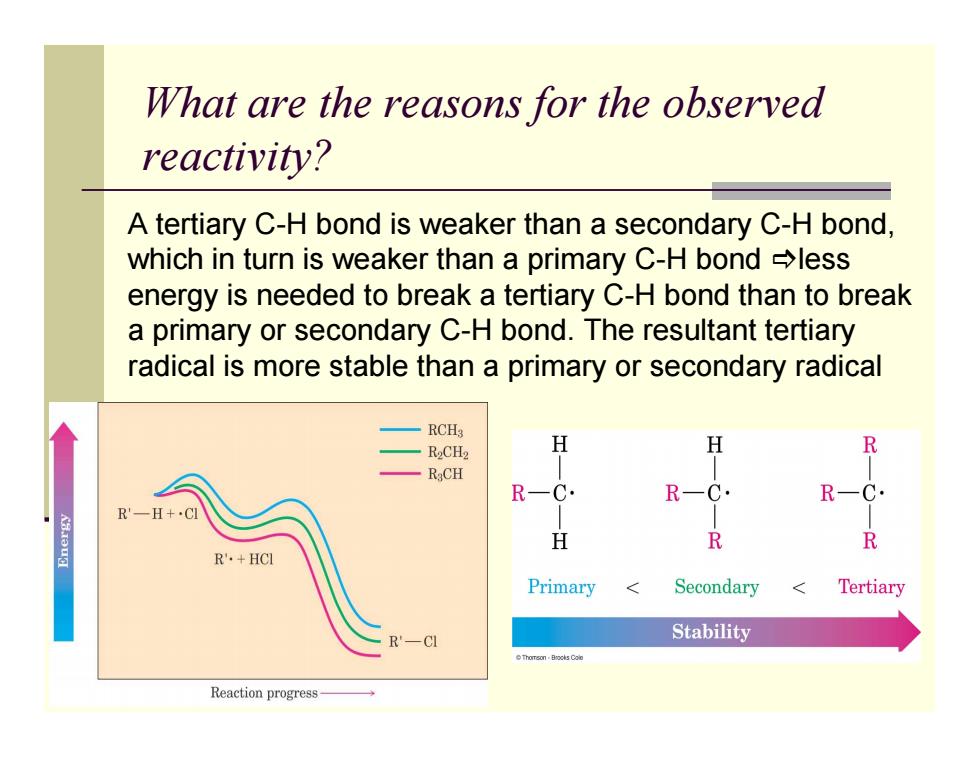
What are the reasons for the observed reactivity? A tertiary C-H bond is weaker than a secondary C-H bond, which in turn is weaker than a primary C-H bond less energy is needed to break a tertiary C-H bond than to break a primary or secondary C-H bond.The resultant tertiary radical is more stable than a primary or secondary radical 一RCHg -R2CH2 H H R RCH R-C. R R'一H+·CI H R R R'·+HCl Primary Secondary Tertiary -CI Stability Reaction progress What are the reasons for the observed reactivity? A tertiary C-H bond is weaker than a secondary C-H bond, which in turn is weaker than a primary C-H bond @less energy is needed to break a tertiary C-H bond than to break a primary or secondary C-H bond. The resultant tertiary radical is more stable than a primary or secondary radical