正在加载图片...
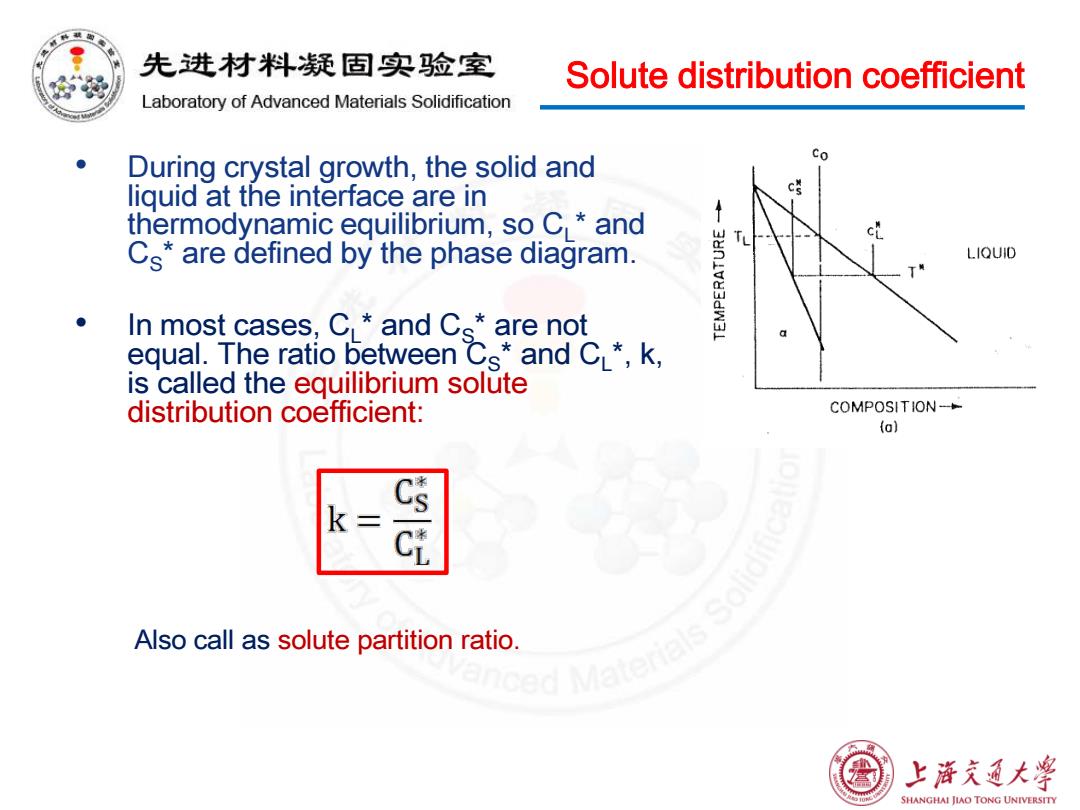
先进材料疑固实验室 Solute distribution coefficient Laboratory of Advanced Materials Solidification During crystal growth,the solid and Co liguid at the interface are in c誉 thermodynamic equilibrium,so C and ↑ cL Cs*are defined by the phase diagram. LIQUID In most cases:CL*and Cs*are not equal.The ratio between Cs*and CL*,k, is called the equilibrium solute distribution coefficient: COMPOSITION- (o) k= Cs Also call as solute partition ratio ced Matenak 上浒充通大学 SHANGHAI JIAO TONG UNIVERSITYSolute distribution coefficient • During crystal growth, the solid and liquid at the interface are in thermodynamic equilibrium, so CL* and CS* are defined by the phase diagram. • In most cases, CL* and CS* are not equal. The ratio between CS* and CL*, k, is called the equilibrium solute distribution coefficient: Also call as solute partition ratio