正在加载图片...
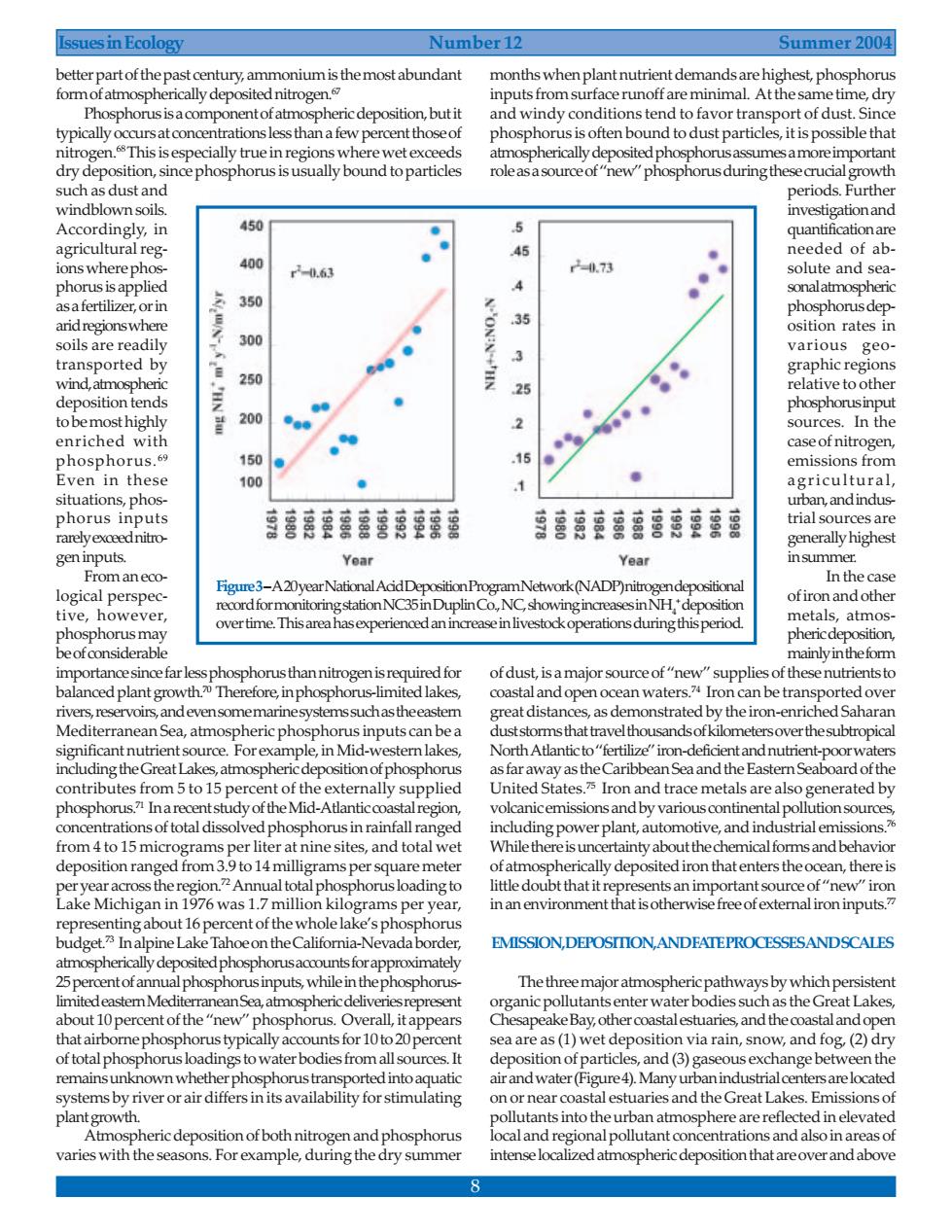
Issues in Ecology Number 12 Summer 2004 betterpartof thepast century,ammonium is themost abundant months whenplant nutrient demands are highest,phosphorus formof atmospherically deposited nitrogen inputs fromsurface runoff are minimal.At the same time,dry Phosphorus is acomponent of atmospheric deposition,buti and windy conditions ter dtofavor und to dust parti tha such as dust and periods.Further windblown soils. Accordingly,in 450 ● agricultu reg 400 45 ded r20.63 .73 and 4 350 arid regionswhere 35 osition rates in soils are readily 30 various geo transported by graphic regions 25 ve to to bemost highly 量200 ●。 enriched with case of nitrogen phosphorus. 150 ● emissions from Even in these 100 1 菌营鹿蓉套富喜落空索雷 菌雪商营喜店草落露 rally highes gen inputs Year Year Fromaneco In the case logical perspec Figure3-A20yearNationalAcdDeposit ofiron and other epo tive, NH metals,atmos phosphorus thannitrogen is required for supplies of these nutrients to balanced plant growth.Therefore,inphosphorus-limited lakes, coastaland open ocean waters.Iron can be transported over rivers,reservoirs,and evensomemarinesystems suchas theeastem great distances,as demonstrated by the iron-enriched Saharar Mediterranea Sea,atmosphericphosphorus inputs can bea gtutiemt contributes from 5to 15 ne of the externally su rated b volcanicemissions and by various continental pollution sources concentrations of total dissolvedphosphorus in rainfall ranged including power plant,automotive,and industrialemissions.? perliter at nine sites,and total wet While thereisuncertainty about the hemicalforms and behavio deposition rangec ms per square mete ally deposited iron thatenters theocean,there i rninaboutperntofhewholelke'sphosphorus budget.Inalpine Lake Tahoeon theCalifornia-Nevada border, EMISSIONDEPOSIIONANDEATEPROCESSESANDSCALES 25percentofannualpho-phorusinputs whi hep The th ajor atmosph icpathways by whichper nt of the enter v es su as sea are as (1)wet den osition via rain,snow,and fog,(2)dry of total phosphorus loadings to water bodies from all sources.It deposition of particles,and (3)gaseous exchange between the remains unknown whether phosphorus transported into aquatic air and water(Figure4).Many urbanindustrialcentersarelocated systems by river or air differs in its availability for stimulating on or near coastal estuaries and the Great Lakes.Emissionso plant growth ants into theu tmosphere are refle varies withthe ericdep osition of both nit P s Fo ample,dur onthiandaoeratr reover and ab 8 Issues in Ecology Number 12 Summer 2004 better part of the past century, ammonium is the most abundant form of atmospherically deposited nitrogen.67 Phosphorus is a component of atmospheric deposition, but it typically occurs at concentrations less than a few percent those of nitrogen.68 This is especially true in regions where wet exceeds dry deposition, since phosphorus is usually bound to particles such as dust and windblown soils. Accordingly, in agricultural regions where phosphorus is applied as a fertilizer, or in arid regions where soils are readily transported by wind, atmospheric deposition tends to be most highly enriched with phosphorus.69 Even in these situations, phosphorus inputs rarely exceed nitrogen inputs. From an ecological perspective, however, phosphorus may be of considerable importance since far less phosphorus than nitrogen is required for balanced plant growth.70 Therefore, in phosphorus-limited lakes, rivers, reservoirs, and even some marine systems such as the eastern Mediterranean Sea, atmospheric phosphorus inputs can be a significant nutrient source. For example, in Mid-western lakes, including the Great Lakes, atmospheric deposition of phosphorus contributes from 5 to 15 percent of the externally supplied phosphorus.71 In a recent study of the Mid-Atlantic coastal region, concentrations of total dissolved phosphorus in rainfall ranged from 4 to 15 micrograms per liter at nine sites, and total wet deposition ranged from 3.9 to 14 milligrams per square meter per year across the region.72 Annual total phosphorus loading to Lake Michigan in 1976 was 1.7 million kilograms per year, representing about 16 percent of the whole lake’s phosphorus budget.73 In alpine Lake Tahoe on the California-Nevada border, atmospherically deposited phosphorus accounts for approximately 25 percent of annual phosphorus inputs, while in the phosphoruslimited eastern Mediterranean Sea, atmospheric deliveries represent about 10 percent of the “new” phosphorus. Overall, it appears that airborne phosphorus typically accounts for 10 to 20 percent of total phosphorus loadings to water bodies from all sources. It remains unknown whether phosphorus transported into aquatic systems by river or air differs in its availability for stimulating plant growth. Atmospheric deposition of both nitrogen and phosphorus varies with the seasons. For example, during the dry summer months when plant nutrient demands are highest, phosphorus inputs from surface runoff are minimal. At the same time, dry and windy conditions tend to favor transport of dust. Since phosphorus is often bound to dust particles, it is possible that atmospherically deposited phosphorus assumes a more important role as a source of “new” phosphorus during these crucial growth periods. Further investigation and quantification are needed of absolute and seasonal atmospheric phosphorus deposition rates in various geographic regions relative to other phosphorus input sources. In the case of nitrogen, emissions from agricultural, urban, and industrial sources are generally highest in summer. In the case of iron and other metals, atmospheric deposition, mainly in the form of dust, is a major source of “new” supplies of these nutrients to coastal and open ocean waters.74 Iron can be transported over great distances, as demonstrated by the iron-enriched Saharan dust storms that travel thousands of kilometers over the subtropical North Atlantic to “fertilize” iron-deficient and nutrient-poor waters as far away as the Caribbean Sea and the Eastern Seaboard of the United States.75 Iron and trace metals are also generated by volcanic emissions and by various continental pollution sources, including power plant, automotive, and industrial emissions.76 While there is uncertainty about the chemical forms and behavior of atmospherically deposited iron that enters the ocean, there is little doubt that it represents an important source of “new” iron in an environment that is otherwise free of external iron inputs.77 EMISSION, DEPOSITION, AND FATE PROCESSES AND SCALES The three major atmospheric pathways by which persistent organic pollutants enter water bodies such as the Great Lakes, Chesapeake Bay, other coastal estuaries, and the coastal and open sea are as (1) wet deposition via rain, snow, and fog, (2) dry deposition of particles, and (3) gaseous exchange between the air and water (Figure 4). Many urban industrial centers are located on or near coastal estuaries and the Great Lakes. Emissions of pollutants into the urban atmosphere are reflected in elevated local and regional pollutant concentrations and also in areas of intense localized atmospheric deposition that are over and above Figure 3– A 20 year National Acid Deposition Program Network (NADP) nitrogen depositional record for monitoring station NC35 in Duplin Co., NC, showing increases in NH4 + deposition over time. This area has experienced an increase in livestock operations during this period