正在加载图片...
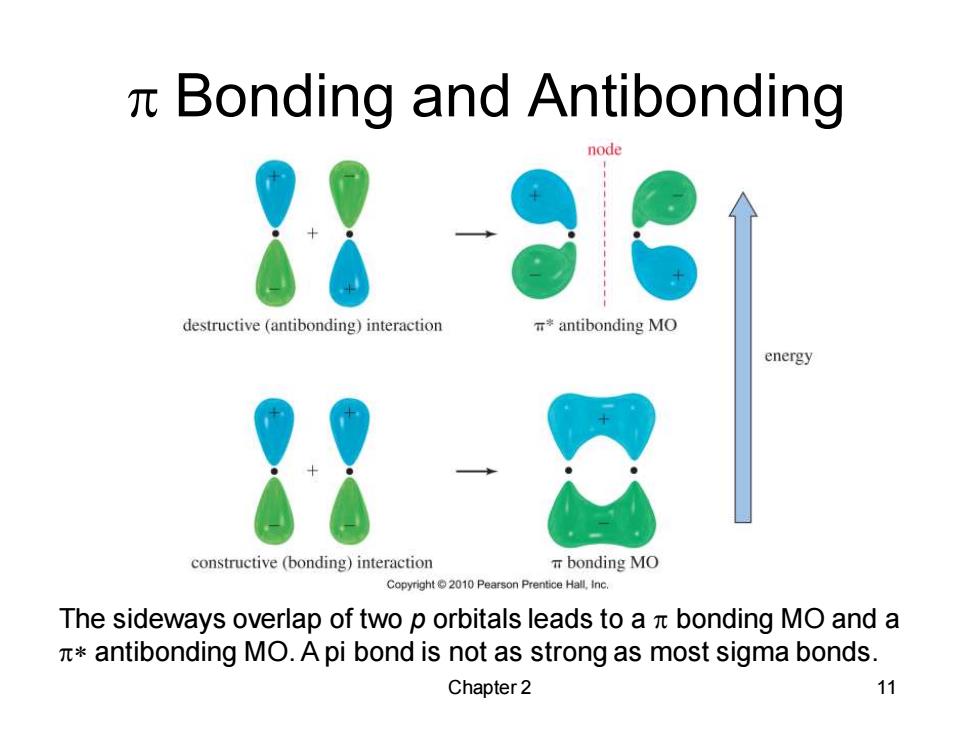
n Bonding and Antibonding node destructive (antibonding)interaction *antibonding MO energy constructive (bonding)interaction bonding MO Copyright 2010 Pearson Prentice Hall,Inc. The sideways overlap of two p orbitals leads to a n bonding MO and a antibonding MO.A pi bond is not as strong as most sigma bonds. Chapter 2 11Chapter 2 11 p Bonding and Antibonding The sideways overlap of two p orbitals leads to a p bonding MO and a p* antibonding MO. A pi bond is not as strong as most sigma bonds