正在加载图片...
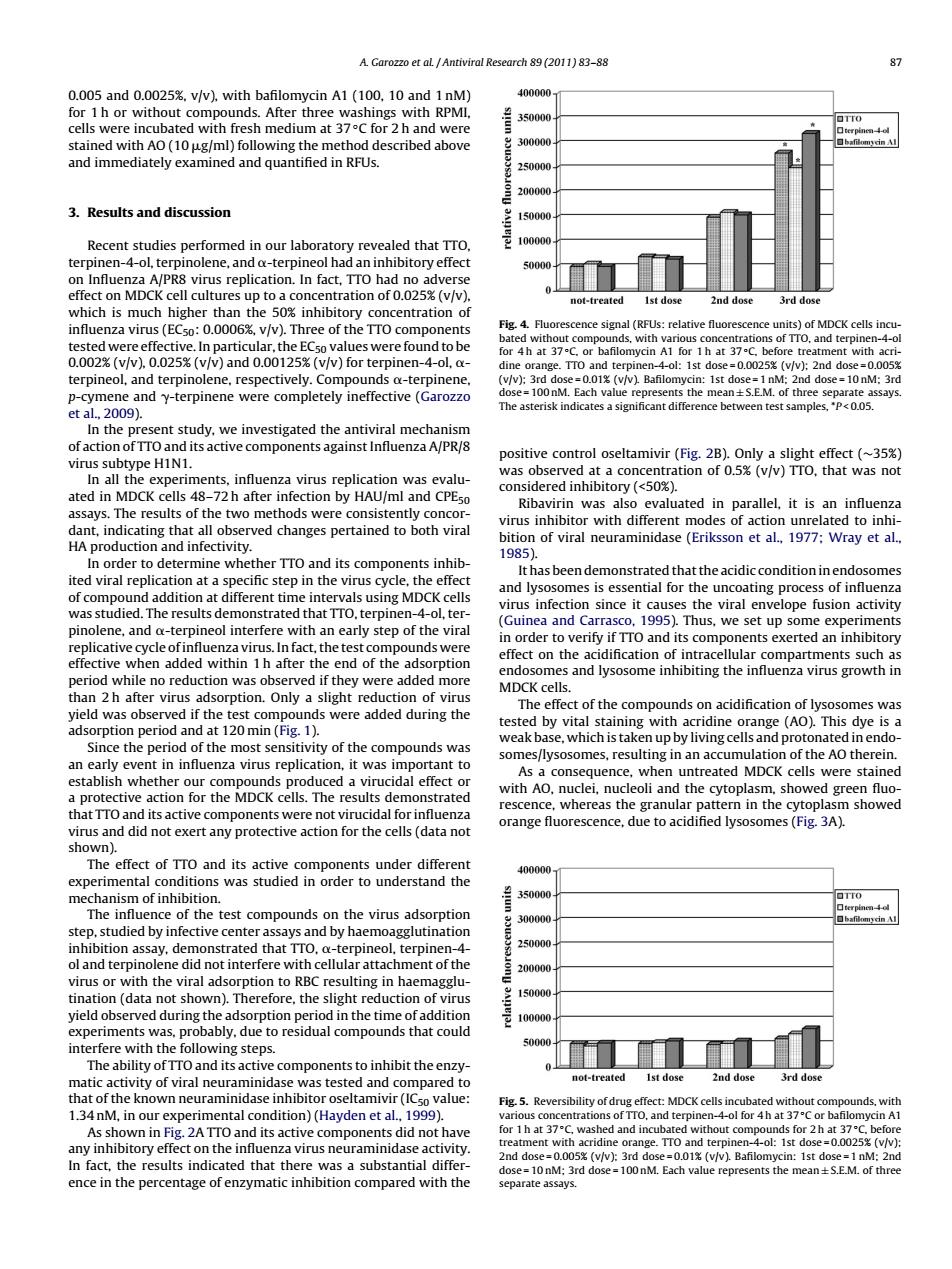
A.Garozzo et al./Antiviral Research 89 (2011)83-88 87 0.005 and 0.0025%,v/v),with bafilomycin A1 (100,10 and 1 nM) 400000 for 1 h or without compounds.After three washings with RPMI 350000 OTTO cells were incubated with fresh medium at 37C for 2 h and were stained with AO(10 ug/ml)following the method described above 300000 ☐hfm3nA and immediately examined and quantified in RFUs. 250000 200000 3.Results and discussion 150000 Recent studies performed in our laboratory revealed that TTO 100000 terpinen-4-ol,terpinolene,and o-terpineol had an inhibitory effect on Influenza A/PR8 virus replication.In fact,TTO had no adverse effect on MDCK cell cultures up to a concentration of 0.025%(v/v). not-treated Ist dose 2nd dose 3rd dose which is much higher than the 50%inhibitory concentration of influenza virus(ECso:0.0006%,v/v).Three of the TTO components Fig.4.Fluorescence signal (RFUs:relative fluorescence units)of MDCK cells incu- tested were effective.In particular,the ECso values were found to be bated without compounds.with various concentrations of TTO.and terpinen-4-ol for 4h at 37C.or bafilomycin A1 for 1 h at 37C.before treatment with acri- 0.002%(v/v),0.025%(v/v)and 0.00125%(v/v)for terpinen-4-ol,a- dine orange.TTO and terpinen-4-ol:1st dose=0.0025%(v/v):2nd dose=0.005% terpineol,and terpinolene,respectively.Compounds o-terpinene, (v/v):3rd dose=0.01%(v/v).Bafilomycin:1st dose=1 nM:2nd dose=10nM:3rd p-cymene and y-terpinene were completely ineffective (Garozzo dose=100nM.Each value represents the mean+S.E.M.of three separate assays. etal,2009). The asterisk indicates a significant difference between test samples,P<0.05. In the present study,we investigated the antiviral mechanism of action of TTO and its active components against Influenza A/PR/8 positive control oseltamivir (Fig.2B).Only a slight effect (~35%) virus subtype H1N1. was observed at a concentration of 0.5%(v/v)TTO,that was not In all the experiments,influenza virus replication was evalu- ated in MDCK cells 48-72h after infection by HAU/ml and CPEso considered inhibitory(<50%). Ribavirin was also evaluated in parallel,it is an influenza assays.The results of the two methods were consistently concor- virus inhibitor with different modes of action unrelated to inhi- dant,indicating that all observed changes pertained to both vira bition of viral neuraminidase(Eriksson et al.,1977:Wray et al., HA production and infectivity. 1985) In order to determine whether TTO and its components inhib- It has been demonstrated that the acidic condition in endosomes ited viral replication at a specific step in the virus cycle,the effect and lysosomes is essential for the uncoating process of influenza of compound addition at different time intervals using MDCK cells virus infection since it causes the viral envelope fusion activity was studied.The results demonstrated that TTO,terpinen-4-ol,ter- (Guinea and Carrasco,1995).Thus,we set up some experiments pinolene,and o-terpineol interfere with an early step of the viral in order to verify if TTO and its components exerted an inhibitory replicative cycle of influenza virus.In fact,the test compounds were effect on the acidification of intracellular compartments such as effective when added within 1 h after the end of the adsorption endosomes and lysosome inhibiting the influenza virus growth in period while no reduction was observed if they were added more MDCK cells. than 2 h after virus adsorption.Only a slight reduction of virus The effect of the compounds on acidification of lysosomes was yield was observed if the test compounds were added during the tested by vital staining with acridine orange(AO).This dye is a adsorption period and at 120 min(Fig.1). weak base,which is taken up by living cells and protonated in endo- Since the period of the most sensitivity of the compounds was somes/lysosomes,resulting in an accumulation of the AO therein. an early event in influenza virus replication,it was important to As a consequence,when untreated MDCK cells were stained establish whether our compounds produced a virucidal effect or with AO,nuclei,nucleoli and the cytoplasm,showed green fluo- a protective action for the MDCK cells.The results demonstrated rescence,whereas the granular pattern in the cytoplasm showed that TTO and its active components were not virucidal for influenza orange fluorescence,due to acidified lysosomes(Fig.3A). virus and did not exert any protective action for the cells(data not shown). The effect of TTO and its active components under different 400000 experimental conditions was studied in order to understand the mechanism of inhibition. 兰 350000 口TT0 The influence of the test compounds on the virus adsorption 30000 step,studied by infective center assays and by haemoagglutination inhibition assay,demonstrated that TTO.o-terpineol,terpinen-4- 250000 ol and terpinolene did not interfere with cellular attachment of the 200000 virus or with the viral adsorption to RBC resulting in haemagglu- tination(data not shown).Therefore,the slight reduction of virus 15000 yield observed during the adsorption period in the time of addition 100000 experiments was,probably,due to residual compounds that could interfere with the following steps. 50000 The ability of TTO and its active components to inhibit the enzy- matic activity of viral neuraminidase was tested and compared to not-treated Ist dose 2nd dose 3rd dose that of the known neuraminidase inhibitor oseltamivir(ICso value: Fig.5.Reversibility of drug effect:MDCK cells incubated without compounds,with 1.34 nM,in our experimental condition)(Hayden et al.,1999). various concentrations of TTO,and terpinen-4-ol for 4h at 37C or bafilomycin A1 As shown in Fig.2A TTO and its active components did not have for 1h at 37C,washed and incubated without compounds for 2h at 37C.before any inhibitory effect on the influenza virus neuraminidase activity treatment with acridine orange.TTO and terpinen-4-ol:1st dose=0.0025%(v/v): 2nd dose=0.005%(v/v):3rd dose=0.01(v/v).Bafilomycin:1st dose=1 nM:2nd In fact,the results indicated that there was a substantial differ- dose=10 nM:3rd dose=100 nM.Each value represents the mean+S.E.M.of three ence in the percentage of enzymatic inhibition compared with the separate assays.A. Garozzo et al. / Antiviral Research 89 (2011) 83–88 87 0.005 and 0.0025%, v/v), with bafilomycin A1 (100, 10 and 1 nM) for 1 h or without compounds. After three washings with RPMI, cells were incubated with fresh medium at 37 ◦C for 2 h and were stained with AO (10 g/ml) following the method described above and immediately examined and quantified in RFUs. 3. Results and discussion Recent studies performed in our laboratory revealed that TTO, terpinen-4-ol, terpinolene, and -terpineol had an inhibitory effect on Influenza A/PR8 virus replication. In fact, TTO had no adverse effect on MDCK cell cultures up to a concentration of 0.025% (v/v), which is much higher than the 50% inhibitory concentration of influenza virus (EC50: 0.0006%, v/v). Three of the TTO components tested were effective. In particular, the EC50 values were found to be 0.002% (v/v), 0.025% (v/v) and 0.00125% (v/v) for terpinen-4-ol, - terpineol, and terpinolene, respectively. Compounds -terpinene, p-cymene and -terpinene were completely ineffective (Garozzo et al., 2009). In the present study, we investigated the antiviral mechanism of action of TTO and its active components against Influenza A/PR/8 virus subtype H1N1. In all the experiments, influenza virus replication was evaluated in MDCK cells 48–72 h after infection by HAU/ml and CPE50 assays. The results of the two methods were consistently concordant, indicating that all observed changes pertained to both viral HA production and infectivity. In order to determine whether TTO and its components inhibited viral replication at a specific step in the virus cycle, the effect of compound addition at different time intervals using MDCK cells was studied. The results demonstrated that TTO, terpinen-4-ol, terpinolene, and -terpineol interfere with an early step of the viral replicative cycle of influenza virus. In fact, the test compounds were effective when added within 1 h after the end of the adsorption period while no reduction was observed if they were added more than 2 h after virus adsorption. Only a slight reduction of virus yield was observed if the test compounds were added during the adsorption period and at 120 min (Fig. 1). Since the period of the most sensitivity of the compounds was an early event in influenza virus replication, it was important to establish whether our compounds produced a virucidal effect or a protective action for the MDCK cells. The results demonstrated that TTO and its active components were not virucidal for influenza virus and did not exert any protective action for the cells (data not shown). The effect of TTO and its active components under different experimental conditions was studied in order to understand the mechanism of inhibition. The influence of the test compounds on the virus adsorption step, studied by infective center assays and by haemoagglutination inhibition assay, demonstrated that TTO, -terpineol, terpinen-4- ol and terpinolene did not interfere with cellular attachment of the virus or with the viral adsorption to RBC resulting in haemagglutination (data not shown). Therefore, the slight reduction of virus yield observed during the adsorption period in the time of addition experiments was, probably, due to residual compounds that could interfere with the following steps. The ability of TTO and its active components to inhibit the enzymatic activity of viral neuraminidase was tested and compared to that of the known neuraminidase inhibitor oseltamivir (IC50 value: 1.34 nM, in our experimental condition) (Hayden et al., 1999). As shown in Fig. 2A TTO and its active components did not have any inhibitory effect on the influenza virus neuraminidase activity. In fact, the results indicated that there was a substantial difference in the percentage of enzymatic inhibition compared with the Fig. 4. Fluorescence signal (RFUs: relative fluorescence units) of MDCK cells incubated without compounds, with various concentrations of TTO, and terpinen-4-ol for 4 h at 37 ◦C, or bafilomycin A1 for 1 h at 37 ◦C, before treatment with acridine orange. TTO and terpinen-4-ol: 1st dose = 0.0025% (v/v); 2nd dose = 0.005% (v/v); 3rd dose = 0.01% (v/v). Bafilomycin: 1st dose = 1 nM; 2nd dose = 10 nM; 3rd dose = 100 nM. Each value represents the mean ± S.E.M. of three separate assays. The asterisk indicates a significant difference between test samples, *P < 0.05. positive control oseltamivir (Fig. 2B). Only a slight effect (∼35%) was observed at a concentration of 0.5% (v/v) TTO, that was not considered inhibitory (<50%). Ribavirin was also evaluated in parallel, it is an influenza virus inhibitor with different modes of action unrelated to inhibition of viral neuraminidase (Eriksson et al., 1977; Wray et al., 1985). It has been demonstrated that the acidic condition in endosomes and lysosomes is essential for the uncoating process of influenza virus infection since it causes the viral envelope fusion activity (Guinea and Carrasco, 1995). Thus, we set up some experiments in order to verify if TTO and its components exerted an inhibitory effect on the acidification of intracellular compartments such as endosomes and lysosome inhibiting the influenza virus growth in MDCK cells. The effect of the compounds on acidification of lysosomes was tested by vital staining with acridine orange (AO). This dye is a weak base, which is taken up by living cells and protonated in endosomes/lysosomes, resulting in an accumulation of the AO therein. As a consequence, when untreated MDCK cells were stained with AO, nuclei, nucleoli and the cytoplasm, showed green fluorescence, whereas the granular pattern in the cytoplasm showed orange fluorescence, due to acidified lysosomes (Fig. 3A). Fig. 5. Reversibility of drug effect: MDCK cells incubated without compounds, with various concentrations of TTO, and terpinen-4-ol for 4 h at 37 ◦C or bafilomycin A1 for 1 h at 37 ◦C, washed and incubated without compounds for 2 h at 37 ◦C, before treatment with acridine orange. TTO and terpinen-4-ol: 1st dose = 0.0025% (v/v); 2nd dose = 0.005% (v/v); 3rd dose = 0.01% (v/v). Bafilomycin: 1st dose = 1 nM; 2nd dose = 10 nM; 3rd dose = 100 nM. Each value represents the mean ± S.E.M. of three separate assays.������