正在加载图片...
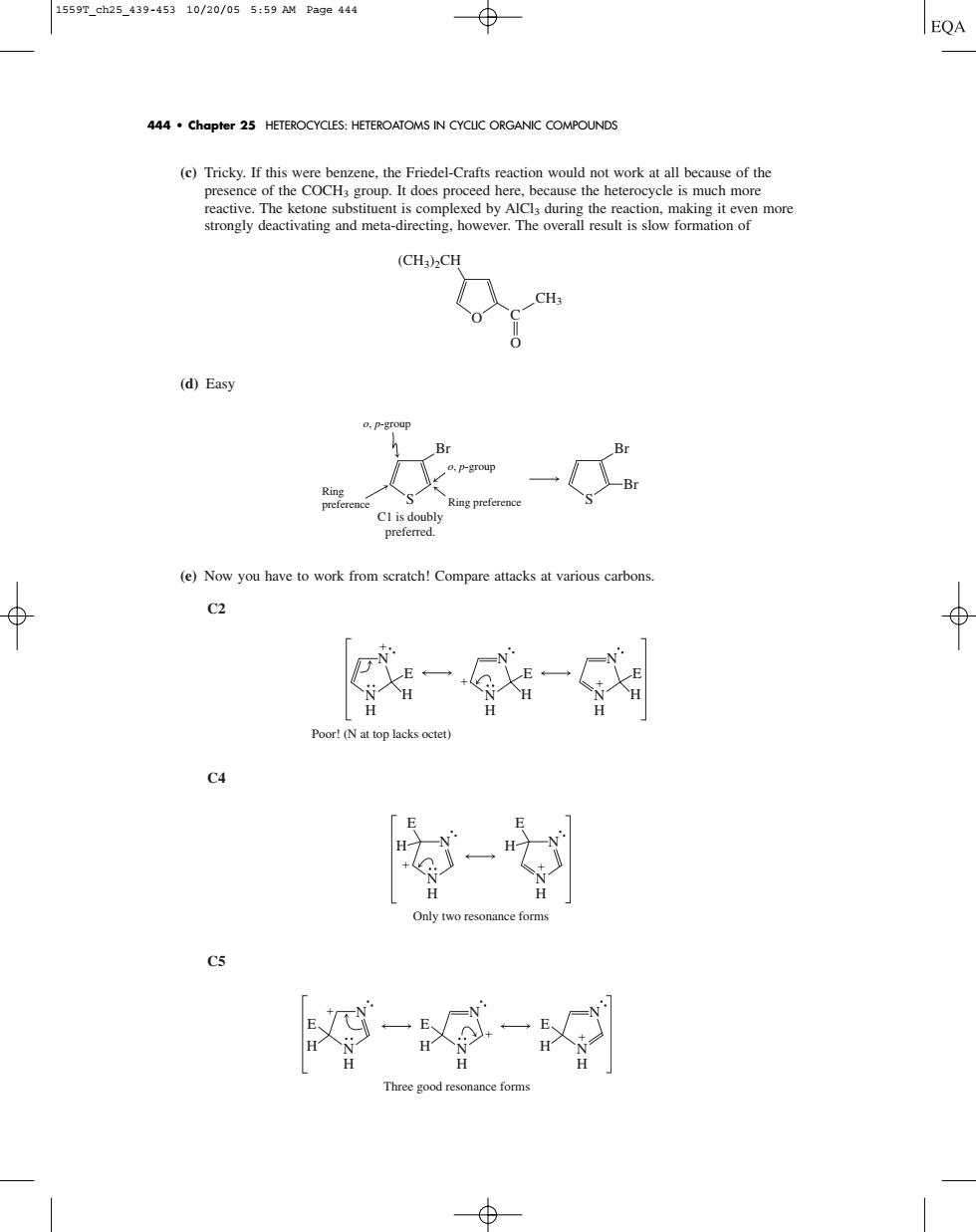
1559T_eh25.439-45310/20/055:59 M Page444 EQA 444.chapter 25 HETEROCYCLES:HETEROATOMS IN CYCLC ORGANIC COMPOUNDS (c)Tricky.If this (CHa)2CH (d)Easy o.P-group Br Ring preferenc (e)Now you have to work from scratch!Compare attacks at various carbons ⊕ Poor!(N at top lacks octe C 9-g ood resonance fom(c) Tricky. If this were benzene, the Friedel-Crafts reaction would not work at all because of the presence of the COCH3 group. It does proceed here, because the heterocycle is much more reactive. The ketone substituent is complexed by AlCl3 during the reaction, making it even more strongly deactivating and meta-directing, however. The overall result is slow formation of (d) Easy (e) Now you have to work from scratch! Compare attacks at various carbons. C2 C4 C5 N N H E H Three good resonance forms N N H E H N N H E H N N H N N H E H E H Only two resonance forms N N H E H N N H E H N N H E H Poor! (N at top lacks octet) Br o, p-group o, p-group Ring preference Ring preference C1 is doubly preferred. S S Br Br C O (CH3)2CH CH3 O 444 • Chapter 25 HETEROCYCLES: HETEROATOMS IN CYCLIC ORGANIC COMPOUNDS 1559T_ch25_439-453 10/20/05 5:59 AM Page 444