正在加载图片...
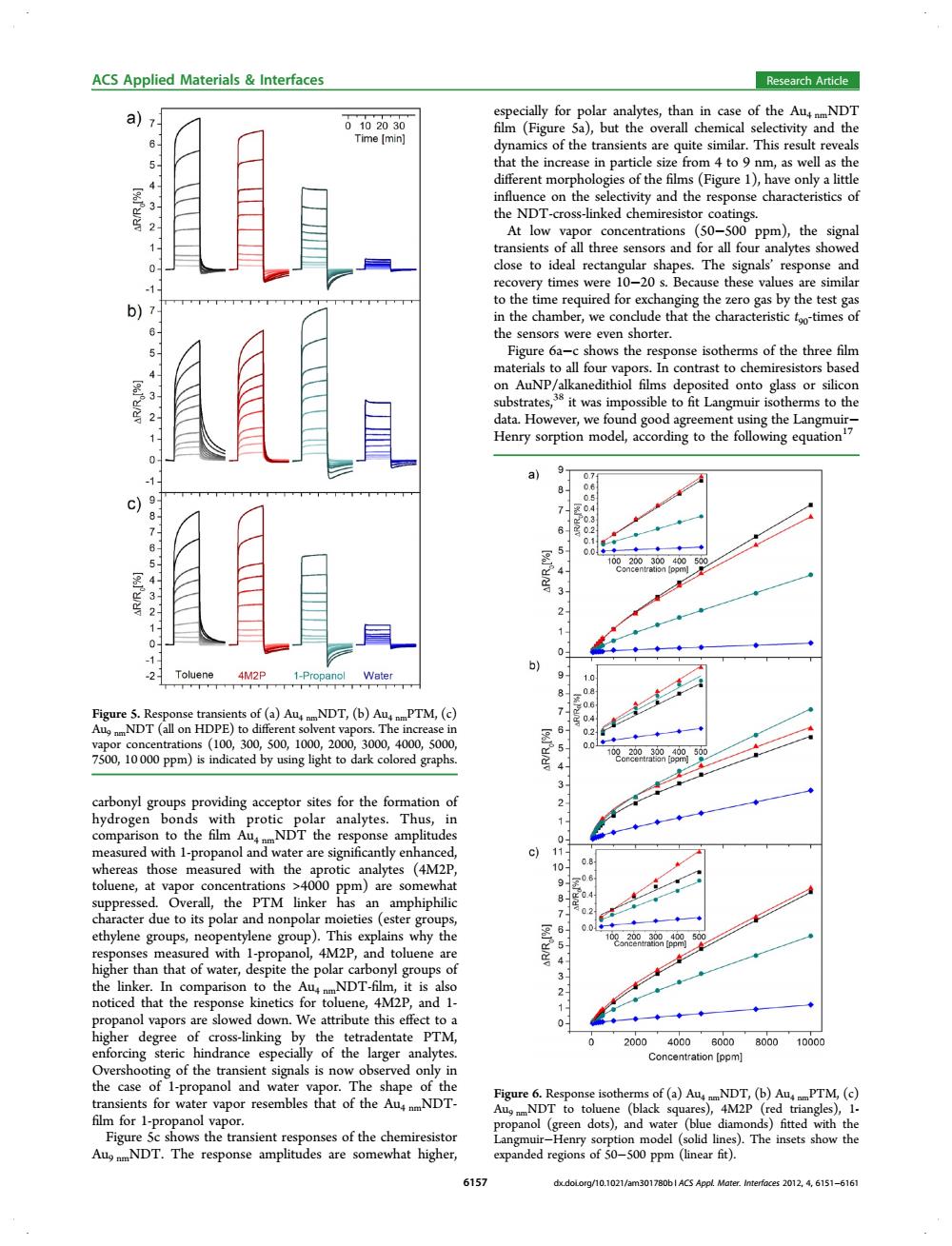
ACS Applied Materials Interfaces Research Article a) especially for polar analytes,than in case of the AuNDT 0102030 film (Figure 5a),but the overall chemical selectivity and the Time [min] dynamics of the transients are quite similar.This result reveals 5、 that the increase in particle size from 4 to 9 nm,as well as the different morphologies of the films(Figure 1),have only a little 432 influence on the selectivity and the response characteristics of the NDT-cross-linked chemiresistor coatings. At low vapor concentrations (50-500 ppm),the signal 1- transients of all three sensors and for all four analytes showed 0 close to ideal rectangular shapes.The signals'response and recovery times were 10-20 s.Because these values are similar to the time required for exchanging the zero gas by the test gas in the chamber,we conclude that the characteristic foo-times of the sensors were even shorter. 5 Figure 6a-c shows the response isotherms of the three film materials to all four vapors.In contrast to chemiresistors based 3 on AuNP/alkanedithiol films deposited onto glass or silicon substrates,38 it was impossible to fit Langmuir isotherms to the 2 data.However,we found good agreement using the Langmuir- Henry sorption model,according to the following equation7 9y 8 7 3 6 0 01 5- 100 4- 3 3 2 2 0- 1 -2 Toluene 4M2P 1-Propanol Water 9. 08 0.6 Figure 5.Response transients of(a)AuNDT,(b)AuPTM,(c) 7 AuNDT(all on HDPE)to different solvent vapors.The increase in 6 0.2 vapor concentrations(100,300,500,1000,2000,3000,4000,5000 0.0 500 7500,10000 ppm)is indicated by using light to dark colored graphs 1oor29tr9400 3 carbonyl groups providing acceptor sites for the formation of 2 hydrogen bonds with protic polar analytes.Thus,in 1 comparison to the film AuNDT the response amplitudes 0 measured with 1-propanol and water are significantly enhanced, c)1 0.8 whereas those measured with the aprotic analytes (4M2P, 10、 toluene,at vapor concentrations >4000 ppm)are somewhat 9 08 suppressed.Overall,the PTM linker has an amphiphilic character due to its polar and nonpolar moieties (ester groups, 7 02 0.0 ethylene groups,neopentylene group).This explains why the 500 responses measured with 1-propanol,4M2P,and toluene are higher than that of water,despite the polar carbonyl groups of 4 the linker.In comparison to the AunmNDT-film,it is also 2 noticed that the response kinetics for toluene,4M2P,and 1- propanol vapors are slowed down.We attribute this effect to a 0 ◆ higher degree of cross-linking by the tetradentate PTM, 2000 40006000800010000 enforcing steric hindrance especially of the larger analytes. Concentration [ppm] Overshooting of the transient signals is now observed only in the case of 1-propanol and water vapor.The shape of the Figure 6.Response isotherms of (a)AumNDT,(b)AumPTM,(c) transients for water vapor resembles that of the AumNDT- Aug NDT to toluene (black squares),4M2P (red triangles),1- film for 1-propanol vapor. propanol (green dots),and water (blue diamonds)fitted with the Figure 5c shows the transient responses of the chemiresistor Langmuir-Henry sorption model (solid lines).The insets show the AumNDT.The response amplitudes are somewhat higher, expanded regions of 50-500 ppm(linear fit). 6157 dx.doLorg/10.1021/am301780bl ACS Appl.Mater.Interfaces 2012,4,6151-6161carbonyl groups providing acceptor sites for the formation of hydrogen bonds with protic polar analytes. Thus, in comparison to the film Au4 nmNDT the response amplitudes measured with 1-propanol and water are significantly enhanced, whereas those measured with the aprotic analytes (4M2P, toluene, at vapor concentrations >4000 ppm) are somewhat suppressed. Overall, the PTM linker has an amphiphilic character due to its polar and nonpolar moieties (ester groups, ethylene groups, neopentylene group). This explains why the responses measured with 1-propanol, 4M2P, and toluene are higher than that of water, despite the polar carbonyl groups of the linker. In comparison to the Au4 nmNDT-film, it is also noticed that the response kinetics for toluene, 4M2P, and 1- propanol vapors are slowed down. We attribute this effect to a higher degree of cross-linking by the tetradentate PTM, enforcing steric hindrance especially of the larger analytes. Overshooting of the transient signals is now observed only in the case of 1-propanol and water vapor. The shape of the transients for water vapor resembles that of the Au4 nmNDT- film for 1-propanol vapor. Figure 5c shows the transient responses of the chemiresistor Au9 nmNDT. The response amplitudes are somewhat higher, especially for polar analytes, than in case of the Au4 nmNDT film (Figure 5a), but the overall chemical selectivity and the dynamics of the transients are quite similar. This result reveals that the increase in particle size from 4 to 9 nm, as well as the different morphologies of the films (Figure 1), have only a little influence on the selectivity and the response characteristics of the NDT-cross-linked chemiresistor coatings. At low vapor concentrations (50−500 ppm), the signal transients of all three sensors and for all four analytes showed close to ideal rectangular shapes. The signals’ response and recovery times were 10−20 s. Because these values are similar to the time required for exchanging the zero gas by the test gas in the chamber, we conclude that the characteristic t90-times of the sensors were even shorter. Figure 6a−c shows the response isotherms of the three film materials to all four vapors. In contrast to chemiresistors based on AuNP/alkanedithiol films deposited onto glass or silicon substrates,38 it was impossible to fit Langmuir isotherms to the data. However, we found good agreement using the Langmuir− Henry sorption model, according to the following equation17 Figure 5. Response transients of (a) Au4 nmNDT, (b) Au4 nmPTM, (c) Au9 nmNDT (all on HDPE) to different solvent vapors. The increase in vapor concentrations (100, 300, 500, 1000, 2000, 3000, 4000, 5000, 7500, 10 000 ppm) is indicated by using light to dark colored graphs. Figure 6. Response isotherms of (a) Au4 nmNDT, (b) Au4 nmPTM, (c) Au9 nmNDT to toluene (black squares), 4M2P (red triangles), 1- propanol (green dots), and water (blue diamonds) fitted with the Langmuir−Henry sorption model (solid lines). The insets show the expanded regions of 50−500 ppm (linear fit). ACS Applied Materials & Interfaces Research Article 6157 dx.doi.org/10.1021/am301780b | ACS Appl. Mater. Interfaces 2012, 4, 6151−6161