正在加载图片...
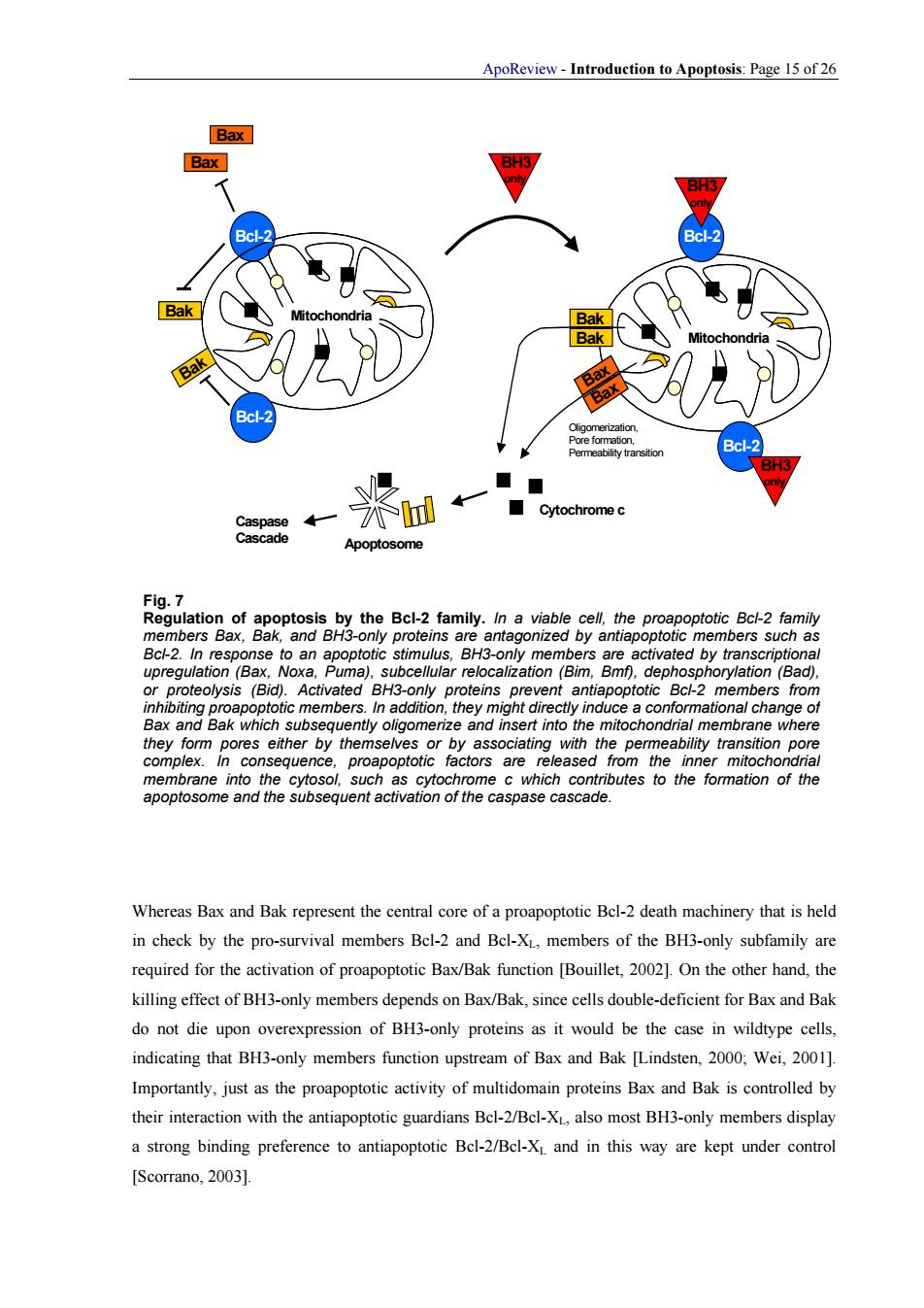
ApoReview-Introduction to Apoptosis:Page 15 of 26 ←← Cytochrome c Apoptosome Bcl-2.In resp vated by transcp of proproapoptotic members inadnins prev mbers fron In additio they form pores either by themselves or by associating with the permeability transition pore such as cytochr apoptosome and the subsequent activation of the caspase cascade. Whereas Bax and Bak represent the central core of a proapoptotic Bel-2 death machinery that is held in check by the pro-survival members Bel-2 and Bcl-X,members of the BH3-only subfamily are required for the activation of proapoptotic Bax/Bak function [the other hand,the killing effect of BH3-only members depends on Bax/Bak,since cells double-deficient for Bax and Bak do not die upon overexpression of BH3-only proteins as it would be the case in wildtype cells, indicating that BH3-only members function upstream of Bax and Bak [Lindsten.2000.Wei.2001] Importantly,just as the proapoptotic activity of multidomain proteins Bax and Bak is controlled by their interaction with the antiapoptotic guardians Bel-2/Bcl-Xalso most BH3-only members display a strong binding preference to antiapoptotic Bel-2/Bcl-X and in this way are kept under control [Scorrano,2003].ApoReview - Introduction to Apoptosis: Page 15 of 26 Mitochondria Mitochondria Bcl-2 Bax Bak BH3 only Bcl-2 BH3 only Bax Bak Bcl-2 Bcl-2 Bak BH3 only Bak Bax Bax Bax Bax Apoptosome Caspase Cascade Cytochrome c Oligomerization, Pore formation, Permeability transition Fig. 7 Regulation of apoptosis by the Bcl-2 family. In a viable cell, the proapoptotic Bcl-2 family members Bax, Bak, and BH3-only proteins are antagonized by antiapoptotic members such as Bcl-2. In response to an apoptotic stimulus, BH3-only members are activated by transcriptional upregulation (Bax, Noxa, Puma), subcellular relocalization (Bim, Bmf), dephosphorylation (Bad), or proteolysis (Bid). Activated BH3-only proteins prevent antiapoptotic Bcl-2 members from inhibiting proapoptotic members. In addition, they might directly induce a conformational change of Bax and Bak which subsequently oligomerize and insert into the mitochondrial membrane where they form pores either by themselves or by associating with the permeability transition pore complex. In consequence, proapoptotic factors are released from the inner mitochondrial membrane into the cytosol, such as cytochrome c which contributes to the formation of the apoptosome and the subsequent activation of the caspase cascade. Whereas Bax and Bak represent the central core of a proapoptotic Bcl-2 death machinery that is held in check by the pro-survival members Bcl-2 and Bcl-XL, members of the BH3-only subfamily are required for the activation of proapoptotic Bax/Bak function [Bouillet, 2002]. On the other hand, the killing effect of BH3-only members depends on Bax/Bak, since cells double-deficient for Bax and Bak do not die upon overexpression of BH3-only proteins as it would be the case in wildtype cells, indicating that BH3-only members function upstream of Bax and Bak [Lindsten, 2000; Wei, 2001]. Importantly, just as the proapoptotic activity of multidomain proteins Bax and Bak is controlled by their interaction with the antiapoptotic guardians Bcl-2/Bcl-XL, also most BH3-only members display a strong binding preference to antiapoptotic Bcl-2/Bcl-XL and in this way are kept under control [Scorrano, 2003]