正在加载图片...
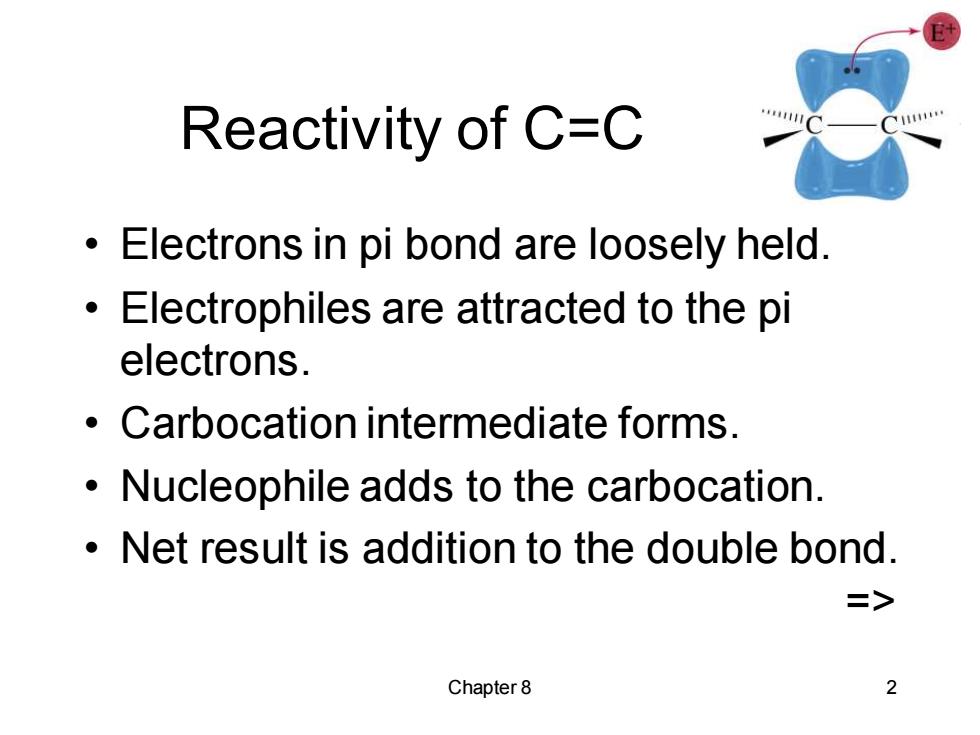
Reactivity of C=C 111 Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. Carbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the double bond. => Chapter 8 2 Chapter 8 2 Reactivity of C=C • Electrons in pi bond are loosely held. • Electrophiles are attracted to the pi electrons. • Carbocation intermediate forms. • Nucleophile adds to the carbocation. • Net result is addition to the double bond. =>