正在加载图片...
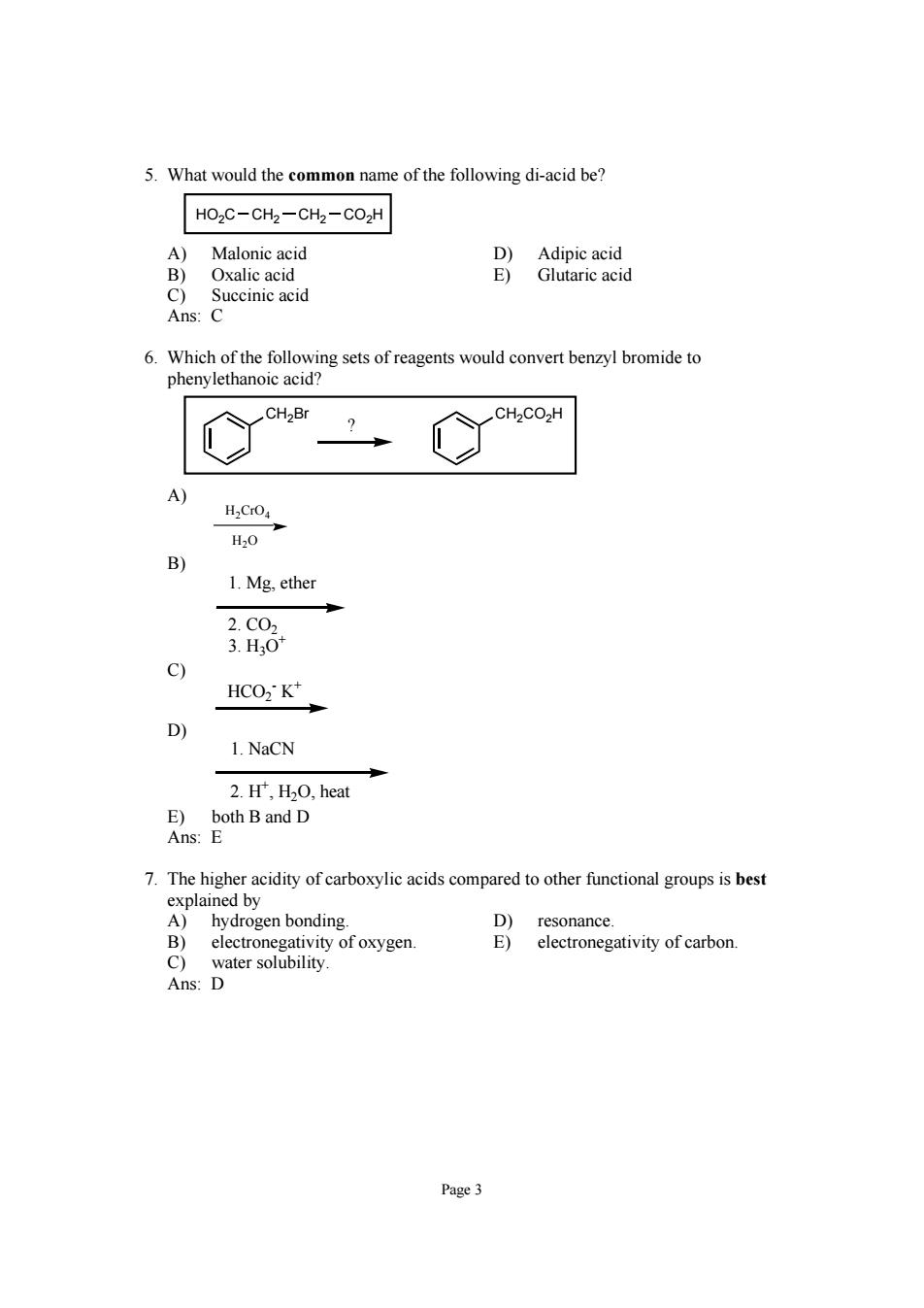
5.What would the common name of the following di-acid be? HOzC-CHz-CH2-CO2H A)Malonic acid Oxalic acid Baneaa Succinic acic 6.Which of the following sets of reagents would convert benzyl bromide to phenylethanoic acid? H.CO H,0 B) 1.Mg.ether 的 c) HCO2K* 1.NaCN 2.H',H2O,heat 7.The higher acidity of carboxylic acids compared to other functional groups is best explained by A)hydrogen bonding. D)resonance. B)electronegativity of oxygen. E)electronegativity of carbon. Page3Page 3 5. What would the common name of the following di-acid be? HO2C CH2 CH2 CO2H A) Malonic acid D) Adipic acid B) Oxalic acid E) Glutaric acid C) Succinic acid Ans: C 6. Which of the following sets of reagents would convert benzyl bromide to phenylethanoic acid? CH2Br CH2CO2H ? A) H2CrO4 H2O B) 1. Mg, ether 2. CO2 3. H3O+ C) HCO2 - K+ D) 1. NaCN 2. H+ , H2O, heat E) both B and D Ans: E 7. The higher acidity of carboxylic acids compared to other functional groups is best explained by A) hydrogen bonding. D) resonance. B) electronegativity of oxygen. E) electronegativity of carbon. C) water solubility. Ans: D