正在加载图片...
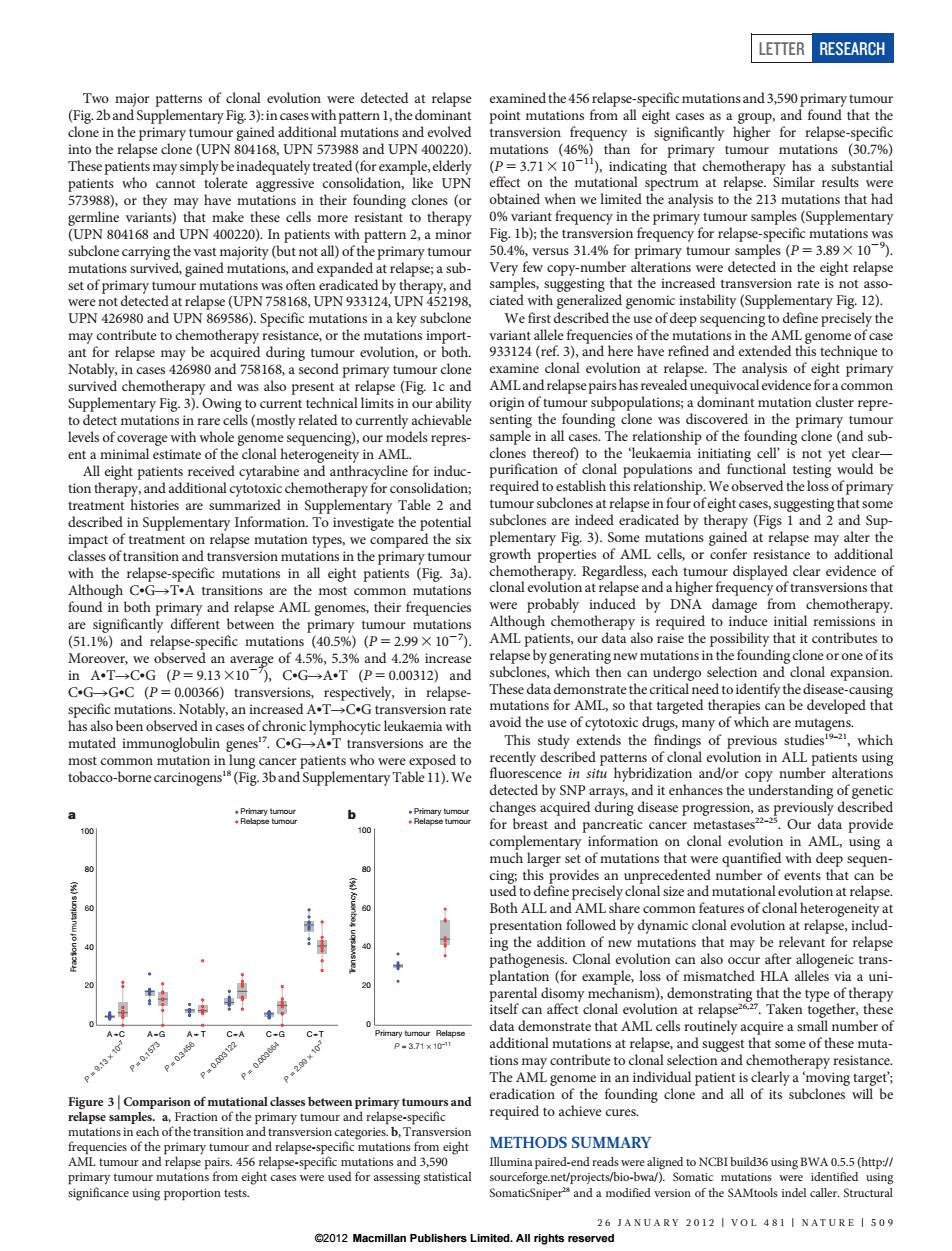
ETTER RESEARCH Two major patterns of clonal evolution were detected at relapse examined the 456 relapse-specific mutations and 3,590 primary tumour (Fig.2band Supplementary Fig.3):in cases with pattern 1,the dominant point mutations from all eight cases as a group,and found that the clone in the primary tumour gained additional mutations and evolved transversion frequency is significantly higher for relapse-specific into the relapse clone(UPN 804168,UPN 573988 and UPN 400220). mutations (46%)than for primary tumour mutations (30.7%) These patients may simply be inadequately treated(for example,elderly (P=3.71X 101),indicating that chemotherapy has a substantial patients who cannot tolerate aggressive consolidation,like UPN effect on the mutational spectrum at relapse.Similar results were 573988),or they may have mutations in their founding clones(or obtained when we limited the analysis to the 213 mutations that had germline variants)that make these cells more resistant to therapy 0%variant frequency in the primary tumour samples(Supplementary (UPN 804168 and UPN 400220).In patients with pattern 2,a minor Fig.Ib);the transversion frequency for relapse-specific mutations was subclone carrying the vast majority (but not all)of the primary tumour 50.4%,versus 31.4%for primary tumour samples (P=3.89 X 10). mutations survived,gained mutations,and expanded at relapse;a sub Very few copy-number alterations were detected in the eight relapse set of primary tumour mutations was often eradicated by therapy,and samples,suggesting that the increased transversion rate is not asso- were not detected at relapse(UPN 758168,UPN 933124,UPN 452198. ciated with generalized genomic instability(Supplementary Fig.12). UPN 426980 and UPN 869586).Specific mutations in a key subclone We first described the use of deep sequencing to define precisely the may contribute to chemotherapy resistance,or the mutations import- variant allele frequencies of the mutations in the AML genome of case ant for relapse may be acquired during tumour evolution,or both. 933124(ref.3),and here have refined and extended this technique to Notably,in cases 426980 and 758168,a second primary tumour clone examine clonal evolution at relapse.The analysis of eight primary survived chemotherapy and was also present at relapse(Fig.Ic and AMLand relapse pairs has revealed unequivocal evidence for a common Supplementary Fig.3).Owing to current technical limits in our ability origin of tumour subpopulations;a dominant mutation cluster repre- to detect mutations in rare cells(mostly related to currently achievable senting the founding clone was discovered in the primary tumour levels of coverage with whole genome sequencing),our models repres- sample in all cases.The relationship of the founding clone (and sub- ent a minimal estimate of the clonal heterogeneity in AML. clones thereof)to the 'leukaemia initiating cell'is not yet clear- All eight patients received cytarabine and anthracycline for induc- purification of clonal populations and functional testing would be tion therapy,and additional cytotoxic chemotherapy for consolidation; required to establish this relationship.We observed the loss of primary treatment histories are summarized in Supplementary Table 2 and tumour subclones at relapse in four ofeight cases,suggesting that some described in Supplementary Information.To investigate the potential subclones are indeed eradicated by therapy(Figs 1 and 2 and Sup- impact of treatment on relapse mutation types,we compared the six plementary Fig.3).Some mutations gained at relapse may alter the classes of transition and transversion mutations in the primary tumour growth properties of AML cells,or confer resistance to additional with the relapse-specific mutations in all eight patients (Fig.3a). chemotherapy.Regardless,each tumour displayed clear evidence of Although C.G>T.A transitions are the most common mutations clonal evolution at relapse and a higher frequency of transversions that found in both primary and relapse AML genomes,their frequencies were probably induced by DNA damage from chemotherapy. are significantly different between the primary tumour mutations Although chemotherapy is required to induce initial remissions in (51.1%)and relapse-specific mutations (40.5%)(P=2.99 X 10-7). AML patients,our data also raise the possibility that it contributes to Moreover,we observed an average of 4.5%,5.3%and 4.2%increase relapse by generating new mutations in the founding clone or one ofits inAT-→CG(P=9.13×10),CG→AT(P=0.00312)and subclones,which then can undergo selection and clonal expansion. C.GG.C (P=0.00366)transversions,respectively,in relapse- These data demonstrate the critical need to identify the disease-causing specific mutations.Notably,an increased A.TC.G transversion rate mutations for AML,so that targeted therapies can be developed that has also been observed in cases of chronic lymphocytic leukaemia with avoid the use of cytotoxic drugs,many of which are mutagens. mutated immunoglobulin genes.C.GA.T transversions are the This study extends the findings of previous studiest-2,which most common mutation in lung cancer patients who were exposed to recently described patterns of clonal evolution in ALL patients using tobacco-borne carcinogens's(Fig.3band Supplementary Table 11).We fluorescence in situ hybridization and/or copy number alterations detected by SNP arrays,and it enhances the understanding of genetic 6 changes acquired during disease progression,as previously described 100 for breast and pancreatic cancer metastases-.Our data provide complementary information on clonal evolution in AML,using a much larger set of mutations that were quantified with deep sequen- 80 90 cing;this provides an unprecedented number of events that can be used to define precisely clonal size and mutational evolution at relapse. 60 Both ALL and AML share common features of clonal heterogeneity at presentation followed by dynamic clonal evolution at relapse,includ- ing the addition of new mutations that may be relevant for relapse pathogenesis.Clonal evolution can also occur after allogeneic trans- plantation (for example,loss of mismatched HLA alleles via a uni- parental disomy mechanism),demonstrating that the type of therapy itself can affect clonal evolution at relapse2.Taken together,these data demonstrate that AML cells routinely acquire a small number of A-C A A.T C.A C.G Primary tumour Relapse Pw0157 P-3.71x10-1 additional mutations at relapse,and suggest that some of these muta- P0003 P200 tions may contribute to clonal selection and chemotherapy resistance. The AML genome in an individual patient is clearly a 'moving target'; Figure 3 Comparison of mutational classes between primary tumours and eradication of the founding clone and all of its subclones will be relapse samples.a,Fraction of the primary tumour and relapse-specific required to achieve cures. mutations in each of the transition and transversion categories.b,Transversion frequencies of the primary tumour and relapse-specific mutations from eight METHODS SUMMARY AML tumour and relapse pairs.456 relapse-specific mutations and 3,590 Illumina paired-end reads were aligned to NCBI build36 using BWA 0.5.5(http:// primary tumour mutations from eight cases were used for assessing statistical sourceforge.net/projects/bio-bwa/).Somatic mutations were identified using significance using proportion tests. SomaticSniper and a modified version of the SAMtools indel caller.Structural 26 JANUARY 2012 VOL 481 NATURE 509 2012 Macmillan Publishers Limited.All rights reservedTwo major patterns of clonal evolution were detected at relapse (Fig. 2b and Supplementary Fig. 3): in caseswith pattern 1, the dominant clone in the primary tumour gained additional mutations and evolved into the relapse clone (UPN 804168, UPN 573988 and UPN 400220). These patients may simply be inadequately treated (for example, elderly patients who cannot tolerate aggressive consolidation, like UPN 573988), or they may have mutations in their founding clones (or germline variants) that make these cells more resistant to therapy (UPN 804168 and UPN 400220). In patients with pattern 2, a minor subclone carrying the vast majority (but not all) of the primary tumour mutations survived, gained mutations, and expanded at relapse; a subset of primary tumour mutations was often eradicated by therapy, and were not detected at relapse (UPN 758168, UPN 933124, UPN 452198, UPN 426980 and UPN 869586). Specific mutations in a key subclone may contribute to chemotherapy resistance, or the mutations important for relapse may be acquired during tumour evolution, or both. Notably, in cases 426980 and 758168, a second primary tumour clone survived chemotherapy and was also present at relapse (Fig. 1c and Supplementary Fig. 3). Owing to current technical limits in our ability to detect mutations in rare cells (mostly related to currently achievable levels of coverage with whole genome sequencing), our models represent a minimal estimate of the clonal heterogeneity in AML. All eight patients received cytarabine and anthracycline for induction therapy, and additional cytotoxic chemotherapy for consolidation; treatment histories are summarized in Supplementary Table 2 and described in Supplementary Information. To investigate the potential impact of treatment on relapse mutation types, we compared the six classes of transition and transversion mutations in the primary tumour with the relapse-specific mutations in all eight patients (Fig. 3a). Although CNGRTNA transitions are the most common mutations found in both primary and relapse AML genomes, their frequencies are significantly different between the primary tumour mutations (51.1%) and relapse-specific mutations (40.5%) (P 5 2.993 1027 ). Moreover, we observed an average of 4.5%, 5.3% and 4.2% increase in ANTRCNG (P 5 9.1331027 ), CNGRANT (P 5 0.00312) and CNGRGNC (P 5 0.00366) transversions, respectively, in relapsespecific mutations. Notably, an increased ANTRCNG transversion rate has also been observed in cases of chronic lymphocytic leukaemia with mutated immunoglobulin genes17. CNGRANT transversions are the most common mutation in lung cancer patients who were exposed to tobacco-borne carcinogens18 (Fig. 3b and Supplementary Table 11).We examined the 456 relapse-specific mutations and 3,590 primary tumour point mutations from all eight cases as a group, and found that the transversion frequency is significantly higher for relapse-specific mutations (46%) than for primary tumour mutations (30.7%) (P 5 3.713 10211), indicating that chemotherapy has a substantial effect on the mutational spectrum at relapse. Similar results were obtained when we limited the analysis to the 213 mutations that had 0% variant frequency in the primary tumour samples (Supplementary Fig. 1b); the transversion frequency for relapse-specific mutations was 50.4%, versus 31.4% for primary tumour samples (P 5 3.893 1029 ). Very few copy-number alterations were detected in the eight relapse samples, suggesting that the increased transversion rate is not associated with generalized genomic instability (Supplementary Fig. 12). We first described the use of deep sequencing to define precisely the variant allele frequencies of the mutations in the AML genome of case 933124 (ref. 3), and here have refined and extended this technique to examine clonal evolution at relapse. The analysis of eight primary AML and relapse pairs has revealed unequivocal evidence for a common origin of tumour subpopulations; a dominant mutation cluster representing the founding clone was discovered in the primary tumour sample in all cases. The relationship of the founding clone (and subclones thereof) to the ‘leukaemia initiating cell’ is not yet clear— purification of clonal populations and functional testing would be required to establish this relationship. We observed the loss of primary tumour subclones at relapse in four of eight cases, suggesting that some subclones are indeed eradicated by therapy (Figs 1 and 2 and Supplementary Fig. 3). Some mutations gained at relapse may alter the growth properties of AML cells, or confer resistance to additional chemotherapy. Regardless, each tumour displayed clear evidence of clonal evolution at relapse and a higher frequency of transversions that were probably induced by DNA damage from chemotherapy. Although chemotherapy is required to induce initial remissions in AML patients, our data also raise the possibility that it contributes to relapse by generating new mutations in the founding clone or one of its subclones, which then can undergo selection and clonal expansion. These data demonstrate the critical need to identify the disease-causing mutations for AML, so that targeted therapies can be developed that avoid the use of cytotoxic drugs, many of which are mutagens. This study extends the findings of previous studies19–21, which recently described patterns of clonal evolution in ALL patients using fluorescence in situ hybridization and/or copy number alterations detected by SNP arrays, and it enhances the understanding of genetic changes acquired during disease progression, as previously described for breast and pancreatic cancer metastases22–25. Our data provide complementary information on clonal evolution in AML, using a much larger set of mutations that were quantified with deep sequencing; this provides an unprecedented number of events that can be used to define precisely clonal size and mutational evolution at relapse. Both ALL and AML share common features of clonal heterogeneity at presentation followed by dynamic clonal evolution at relapse, including the addition of new mutations that may be relevant for relapse pathogenesis. Clonal evolution can also occur after allogeneic transplantation (for example, loss of mismatched HLA alleles via a uniparental disomy mechanism), demonstrating that the type of therapy itself can affect clonal evolution at relapse26,27. Taken together, these data demonstrate that AML cells routinely acquire a small number of additional mutations at relapse, and suggest that some of these mutations may contribute to clonal selection and chemotherapy resistance. The AML genome in an individual patient is clearly a ‘moving target’; eradication of the founding clone and all of its subclones will be required to achieve cures. METHODS SUMMARY Illumina paired-end reads were aligned to NCBI build36 using BWA 0.5.5 (http:// sourceforge.net/projects/bio-bwa/). Somatic mutations were identified using SomaticSniper28 and a modified version of the SAMtools indel caller. Structural Transversion frequency (%) 0 20 40 60 80 100 Primary tumour Relapse tumour Primary tumour Relapse tumour b Primary tumour Relapse P = 3.71 × 10–11 Fraction of mutations (%) 0 20 40 60 80 100 a P = 9.13 × 10–7 A C P = 0.1573 A G P = 0.3456 A T P = 0.003122 C A P = 0.003664 C G P = 2.99 × 10–7 C T Figure 3 | Comparison of mutational classes between primary tumours and relapse samples. a, Fraction of the primary tumour and relapse-specific mutations in each of the transition and transversion categories. b, Transversion frequencies of the primary tumour and relapse-specific mutations from eight AML tumour and relapse pairs. 456 relapse-specific mutations and 3,590 primary tumour mutations from eight cases were used for assessing statistical significance using proportion tests. LETTER RESEARCH 26 JANUARY 2012 | VOL 481 | NATURE | 509 ©2012 Macmillan Publishers Limited. All rights reserved