正在加载图片...
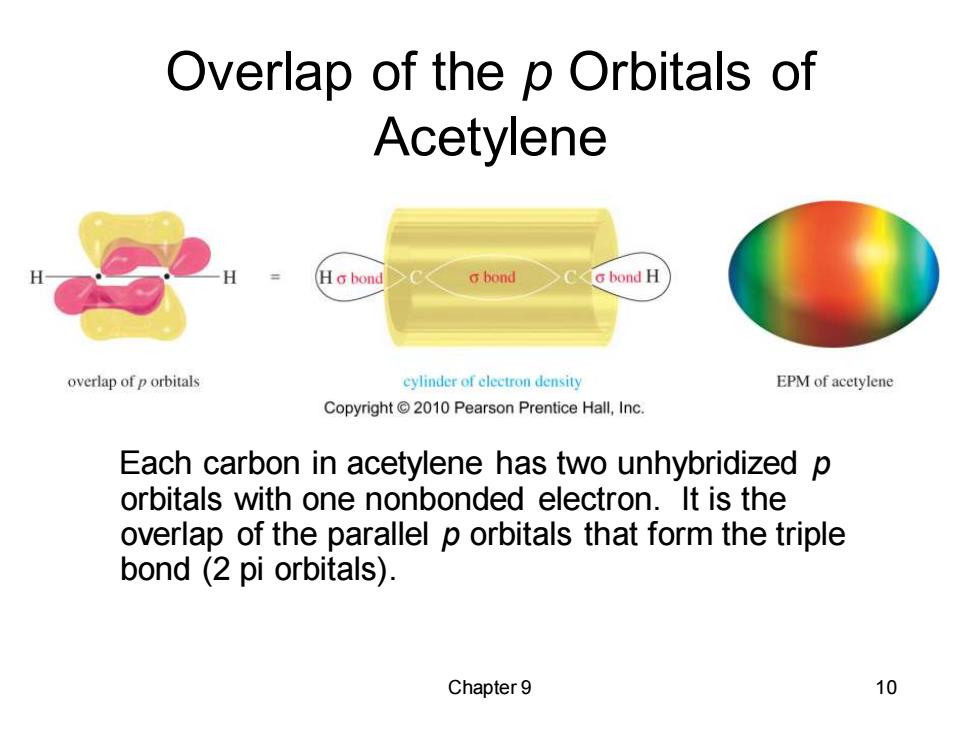
Overlap of the p Orbitals of Acetylene H Ho bond o bond G bond H overlap of p orbitals cylinder of clectron density EPM of acetylene Copyright 2010 Pearson Prentice Hall,Inc. Each carbon in acetylene has two unhybridized p orbitals with one nonbonded electron.It is the overlap of the parallel p orbitals that form the triple bond (2 pi orbitals). Chapter 9 10 Chapter 9 10 Overlap of the p Orbitals of Acetylene Each carbon in acetylene has two unhybridized p orbitals with one nonbonded electron. It is the overlap of the parallel p orbitals that form the triple bond (2 pi orbitals)