正在加载图片...
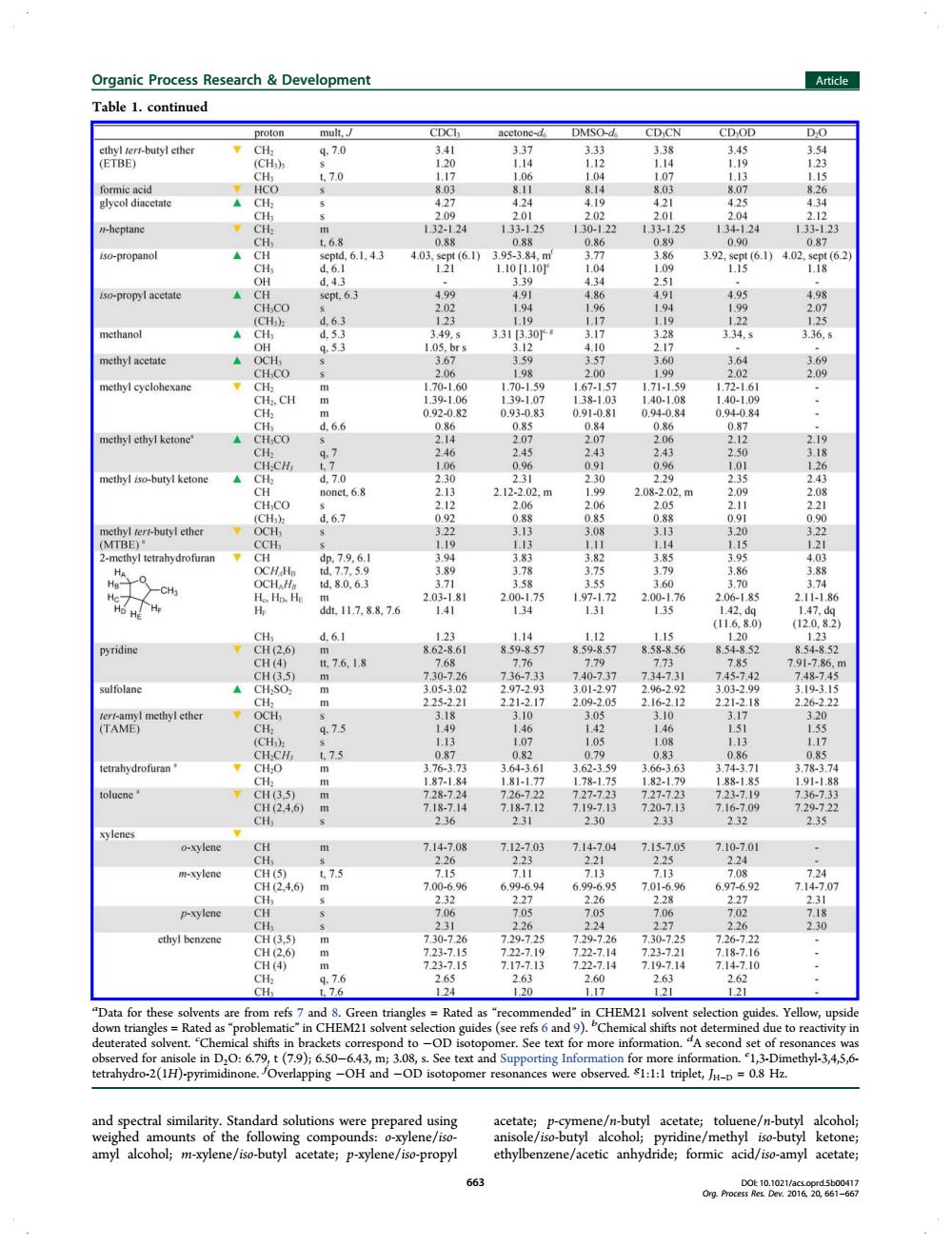
Organic Process Research Development Article Table 1.continued proton mult,J CDCI acetone-d DMSO-d CDCN CDOD ethyl tert-butyl ether CH q.7.0 3.41 3.37 333 338 3.45 3.54 (ETBE) (CH方 120 1.14 1.12 114 1.19 123 CHs t.7.0 1.17 1.06 1.04 1.07 1.13 115 formic acid HCO 8.03 8.11 8.14 8.03 8.07 826 glycol diacetate ACH2 4.27 4.24 4.19 4.21 4.25 4.34 2.09 2.01 2.02 2.01 2.04 2.12 n-heptane 。CH 1.32-1.24 1.33-1.25 130-122 1.33-1.25 1.34-1.24 1.33-123 CH t6.8 0.88 0.88 0.86 0.89 0.90 0.87 iso-propanol ▲CH septd.6.1.4.3 4.03.spt(6.1)3.95-3.84.m 3.77 3.86 3.92.sept(6.1)4.02.sept(6.2) CHy d6.1 121 L.10[1.10 1.04 1.09 1.15 1.18 OH d.4.3 339 4.34 2.51 iso-propyl acetate sept,6.3 4.99 4.91 4.86 4.91 4.95 4.98 CH CO 2.02 1.94 196 194 1.99 2.07 (CH d.6.3 123 1.19 1.17 1.19 122 125 methanol ACHa d.53 349.s 3313.30 3.17 3.28 334,s 3.36.s OH 95.3 1.05,brs 3.12 4.10 2.17 methyl acetate A OCH 3.67 359 357 3.60 3.64 369 CHCO 5 2.06 1.98 2.00 1.99 2.02 2.09 methyl cyclohexane CH: m 1.70-1.60 1.70-1.59 1.67-1.57 1.71-1.59 1.72-1.61 CH2.CH m 1.39-1.06 1.39.1.07 1.38-1.03 1.40-1.08 1.40-1.09 CHa 0.92-0.82 0.93-0.83 0.91-0.81 0.94-0.84 0.94-0.84 CHy d6.6 0.86 0.85 0.84 086 087 methyl ethyl ketone" A CHCO 214 2.07 2.07 2.06 2.12 2.19 CH: q.7 2.46 2.45 243 243 250 3.18 CHCH 47 1.06 0.96 0.91 0.96 1.01 126 methyl iso-butyl ketone A CH2 d.7.0 2.30 2.31 2.30 2.29 2.35 2.43 CH nonet.6.8 2.13 2.12-2.02.m 1.99 2.08-2.02.m 2.09 2.08 CHCO 2.12 2.06 206 2.05 2.11 2.21 (CH3 d.6.7 0.92 0.88 0.85 0.88 0.91 0.90 methyl tert-butyl ether OCH: 322 3.13 3.08 3.13 320 322 (MTBE) CCH 1.19 1.13 1.11 1.14 1.15 1.21 2-methyl tetrahydrofuran CH dp,7.9,6.1 304 387 382 3.85 3.95 4023 Ha OCH Ha td,7.7.5.9 3.89 3.78 3.75 3.79 3.86 3.88 Ha- OCHAH td.80.6.3 371 35父 35 3.60 3.70 374 Ho.Ho.He m 2.03-1.81 2.00-1.75 1.97-1.72 2.00-1.76 2.06-1.85 2.11-1.86 h ddL.11.7.8.8.7.6 1.41 134 131 1.35 1.42.dq 147.dq (11.6.8.0) (12.0.8.2) dn6.1 123 1.14 1.12 1.15 1.20 12 pyridine tCH(2.6) 销 8.62-8.61 8.59-8.57 859-8.57 8.58-8.56 8.548.52 8.54-8.52 CH(4) t7.6.1.8 7.68 7.76 7.79 7.73 7.85 7.91-7.86.m CH(3.5) 730-7.26 7.36-7.33 740-7.37 7.34-731 7.45-742 7.48-7.45 sulfolane m 3.05-3.02 2.97-2.93 3.01-2.97 2.96-2.92 3.03-2.99 3.193.15 CH m 2.25-2.21 2.21-2.17 2.09.2.05 2.16-2.12 2.21-2.18 2.26-2.22 tert-amyl methyl ether OCH 5 3.18 3.10 3.05 3.10 3.17 320 (TAME) CH 9.7.5 1.49 146 142 1.46 1.51 155 (CH) S 1.13 1.07 1.05 1.08 1.13 1.17 CH.CH t7.5 087 0.82 0.79 081 0.86 0R5 tetrahydrofuran ,CH,0 m 3.76-3.73 3.64-3.61 3.62-3.59 3.66-3.63 3.74-3.71 3.78-3.74 CH m 1.87-1.84 181-1.77 1.78-1.75 1.82-1.79 1.88-185 1.91-188 toluene 7CH(3.5) m 7.28-7.24 7,26-722 7.27-723 727.723 7.23-7.19 7.36-7:33 CH(2,4,6) 7.18-714 7.18-7.12 7.19-7.13 7.20-7.13 7.16-7.09 7,29-722 CH 2.36 2.31 2.30 2.33 2.32 2.35 xylenes o-xylene CH m 7.14-7.08 7.12-7.03 7.14-7.04 7.15-7.05 7.10-7.01 CH 2.26 2.23 221 2.25 2.24 m-xylene CH(5) t7.5 7.15 7.11 7.13 7.13 7.08 7.24 CH(2.4.6) m 7.00-6.96 6.99-6.94 6.99-695 7.01-6.96 6.97-6.92 7.147.07 CH 2.32 227 2.26 2.28 2.27 2.31 p-xylene CH 7.06 7.05 7.05 7.06 7.02 7.18 I 231 226 224 227 2.26 2.30 ethyl benzene CH(3.5) m 7.30-7.26 7.29-725 7.29-7.26 7.30-7.25 7.26-7.22 CH(2.6 723.7.15 722.710 722.714 7.23-7.21 7.18-7.16 CH(4) n 723-7.15 7.17-7.13 7.22-7.14 7,19-7.14 7.14-7.10 CH. 9,7.6 2.65 263 60 2.63 2.62 H t7.6 1.24 120 1.17 1.21 121 "Data for these solvents are from refs 7 and 8.Green triangles Rated as "recommended"in CHEM21 solvent selection guides.Yellow,upside down triangles=Rated as'problematic"in CHEM21 solvent selection guides(see refs 6 and 9).Chemical shifts not determined due to reactivity in deuterated solvent.Chemical shifts in brackets correspond to-OD isotopomer.See text for more information."A second set of resonances was observed for anisole in D,O:6.79,t(7.9);6.50-6.43,m;3.08,s.See text and Supporting Information for more information.1,3-Dimethy1-3,4,5,6- tetrahydro-2(1H)-pyrimidinone.Overlapping-OH and-OD isotopomer resonances were observed.1:1:1 triplet,=0.8 Hz. and spectral similarity.Standard solutions were prepared using acetate;p-cymene/n-butyl acetate;toluene/n-butyl alcohol; weighed amounts of the following compounds:o-xylene/iso- anisole/iso-butyl alcohol;pyridine/methyl iso-butyl ketone; amyl alcohol;m-xylene/iso-butyl acetate;p-xylene/iso-propyl ethylbenzene/acetic anhydride;formic acid/iso-amyl acetate; 663 D0t10.1021/acs.oprd5b00417 Org.Process Res.Dev.2016,20,661-667and spectral similarity. Standard solutions were prepared using weighed amounts of the following compounds: o-xylene/isoamyl alcohol; m-xylene/iso-butyl acetate; p-xylene/iso-propyl acetate; p-cymene/n-butyl acetate; toluene/n-butyl alcohol; anisole/iso-butyl alcohol; pyridine/methyl iso-butyl ketone; ethylbenzene/acetic anhydride; formic acid/iso-amyl acetate; Table 1. continued a Data for these solvents are from refs 7 and 8. Green triangles = Rated as “recommended” in CHEM21 solvent selection guides. Yellow, upside down triangles = Rated as “problematic” in CHEM21 solvent selection guides (see refs 6 and 9). b Chemical shifts not determined due to reactivity in deuterated solvent. c Chemical shifts in brackets correspond to −OD isotopomer. See text for more information. d A second set of resonances was observed for anisole in D2O: 6.79, t (7.9); 6.50−6.43, m; 3.08, s. See text and Supporting Information for more information. e 1,3-Dimethyl-3,4,5,6- tetrahydro-2(1H)-pyrimidinone. f Overlapping −OH and −OD isotopomer resonances were observed. g 1:1:1 triplet, JH−D = 0.8 Hz. Organic Process Research & Development Article DOI: 10.1021/acs.oprd.5b00417 Org. Process Res. Dev. 2016, 20, 661−667 663