正在加载图片...
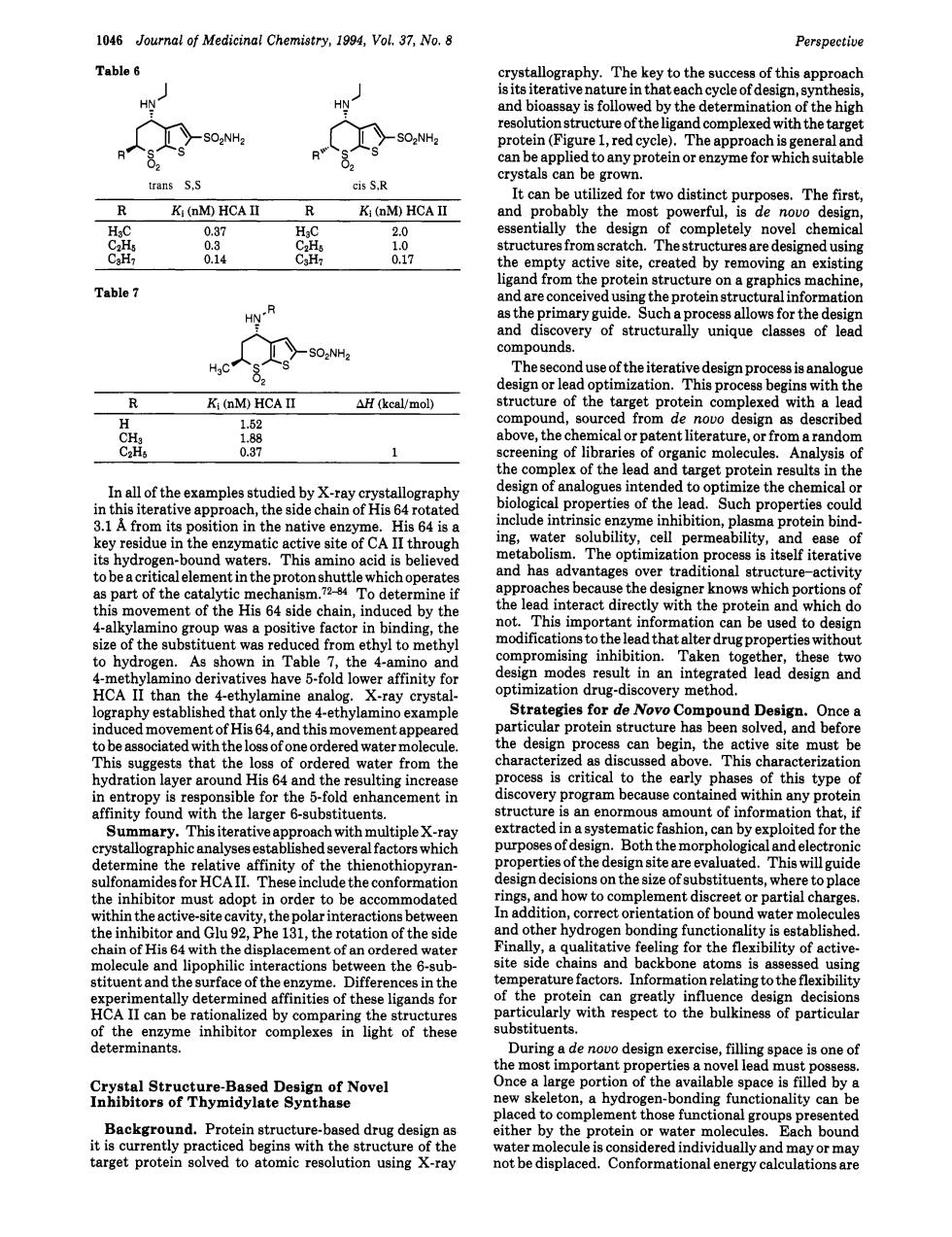
1046 Journal of Medicinal Chemistry,1994,Vol.37,No.8 Perspective Table 6 .The key to theu of this an oac HN is fo ti re of the with the targe ied toany protein or for trans S,S cis S.R 80U Ki (M)HCA II R Ki (DM)HCA II The esse 8 he em oty active site.created by removing an existing Table 7 mthe prote machine Hy月 asheponarye Sucha ery clas The se nd use of the iterative design ign or on.This pro ins with th Ki (nM)HCA I Hcal/mol as described aran of lih of the lead an cal p rties of the l PSuch pr His 64 is the nTanticatnesteofCA lI thro tera kno 0 hich portion 4-akyno tive actor in ter drugp ert 。 in esult in an HCA es have b-told lo e Novo Compound Deg0ce。 can the rom th ss is c ical to the early f this tun y program because e cont ny p ch with ple x. fashion,can by loited for the tallogr ns on th size of substitu wh place 1 r mu adopt dit entation of he inh and Glu92,Phe 131,the rotation of the side ding r lipophilic interactions en the 6-sub ite sid ns and ato n ntally deter da fo deci articula determinants. Soto2etHenaoleDsgaaoNoed portio n of the nality can b ither by the protein mo groupch bound target protein solved to atomic resolution using X-ray not be displaced.Conformational1046 Journal of Medicinal Chemistry, 1994, Vol. 37, No. 8 Table 6 Perspective trans S,S cis S,R R Ki (nM) HCA I1 R Ki (nM) HCA I1 H3C 0.37 H3C 2.0 C2HS 0.3 CZH5 1.0 C3Hl 0.14 C3H7 0.17 Table 7 R Ki (nM) HCA I1 AH (kcal/mol) H 1.52 CH3 1.88 CzHs 0.37 1 In all of the examples studied by X-ray crystallography in this iterative approach, the side chain of His 64 rotated 3.1 A from its position in the native enzyme. His 64 is a key residue in the enzymatic active site of CA I1 through its hydrogen-bound waters. This amino acid is believed to be a critical element in the proton shuttle which operates as part of the catalytic To determine if this movement of the His 64 side chain, induced by the 4-alkylamino group was a positive factor in binding, the size of the substituent was reduced from ethyl to methyl to hydrogen. As shown in Table 7, the 4-amino and 4-methylamino derivatives have 5-fold lower affinity for HCA I1 than the 4-ethylamine analog. X-ray crystallography established that only the 4-ethylamino example induced movement of His 64, and this movement appeared to be associated with the loss of one ordered water molecule. This suggests that the loss of ordered water from the hydration layer around His 64 and the resulting increase in entropy is responsible for the 5-fold enhancement in affinity found with the larger 6-substituents. Summary. This iterative approach with multiple X-ray crystallographic analyses established several factors which determine the relative affinity of the thienothiopyransulfonamides for HCA 11. These include the conformation the inhibitor must adopt in order to be accommodated within the active-site cavity, the polar interactions between the inhibitor and Glu 92, Phe 131, the rotation of the side chain of His 64 with the displacement of an ordered water molecule and lipophilic interactions between the 6-substituent and the surface of the enzyme. Differences in the experimentally determined affinities of these ligands for HCA I1 can be rationalized by comparing the structures of the enzyme inhibitor complexes in light of these determinants. Crystal Structure-Based Design of Novel Inhibitors of Thymidylate Synthase Background. Protein structure-based drug design as it is currently practiced begins with the structure of the target protein solved to atomic resolution using X-ray crystallography. The key to the success of this approach is its iterative nature in that each cycle of design, synthesis, and bioassay is followed by the determination of the high resolution structure of the ligand complexed with the target protein (Figure 1, red cycle). The approach is general and can be applied to any protein or enzyme for which suitable crystals can be grown. It can be utilized for two distinct purposes. The first, and probably the most powerful, is de novo design, essentially the design of completely novel chemical structures from scratch. The structures are designed using the empty active site, created by removing an existing ligand from the protein structure on a graphics machine, and are conceived using the protein structural information as the primary guide. Such a process allows for the design and discovery of structurally unique classes of lead compounds. The second use of the iterative design process is analogue design or lead optimization. This process begins with the structure of the target protein complexed with a lead compound, sourced from de novo design as described above, the chemical or patent literature, or from a random screening of libraries of organic molecules. Analysis of the complex of the lead and target protein results in the design of analogues intended to optimize the chemical or biological properties of the lead. Such properties could include intrinsic enzyme inhibition, plasma protein binding, water solubility, cell permeability, and ease of metabolism. The optimization process is itself iterative and has advantages over traditional structure-activity approaches because the designer knows which portions of the lead interact directly with the protein and which do not. This important information can be used to design modifications to the lead that alter drug properties without compromising inhibition. Taken together, these two design modes result in an integrated lead design and optimization drug-discovery method. Strategies for de Novo Compound Design. Once a particular protein structure has been solved, and before the design process can begin, the active site must be characterized as discussed above. This characterization process is critical to the early phases of this type of discovery program because contained within any protein structure is an enormous amount of information that, if extracted in a systematic fashion, can by exploited for the purposes of design. Both the morphological and electronic properties of the design site are evaluated. This will guide design decisions on the size of substituents, where to place rings, and how to complement discreet or partial charges. In addition, correct orientation of bound water molecules and other hydrogen bonding functionality is established. Finally, a qualitative feeling for the flexibility of activesite side chains and backbone atoms is assessed using temperature factors. Information relating to the flexibility of the protein can greatly influence design decisions particularly with respect to the bulkiness of particular substituents. During a de novo design exercise, filling space is one of the most important properties a novel lead must possess. Once a large portion of the available space is filled by a new skeleton, a hydrogen-bonding functionality can be placed to complement those functional groups presented either by the protein or water molecules. Each bound water molecule is considered individually and may or may not be displaced. Conformational energy calculations are