正在加载图片...
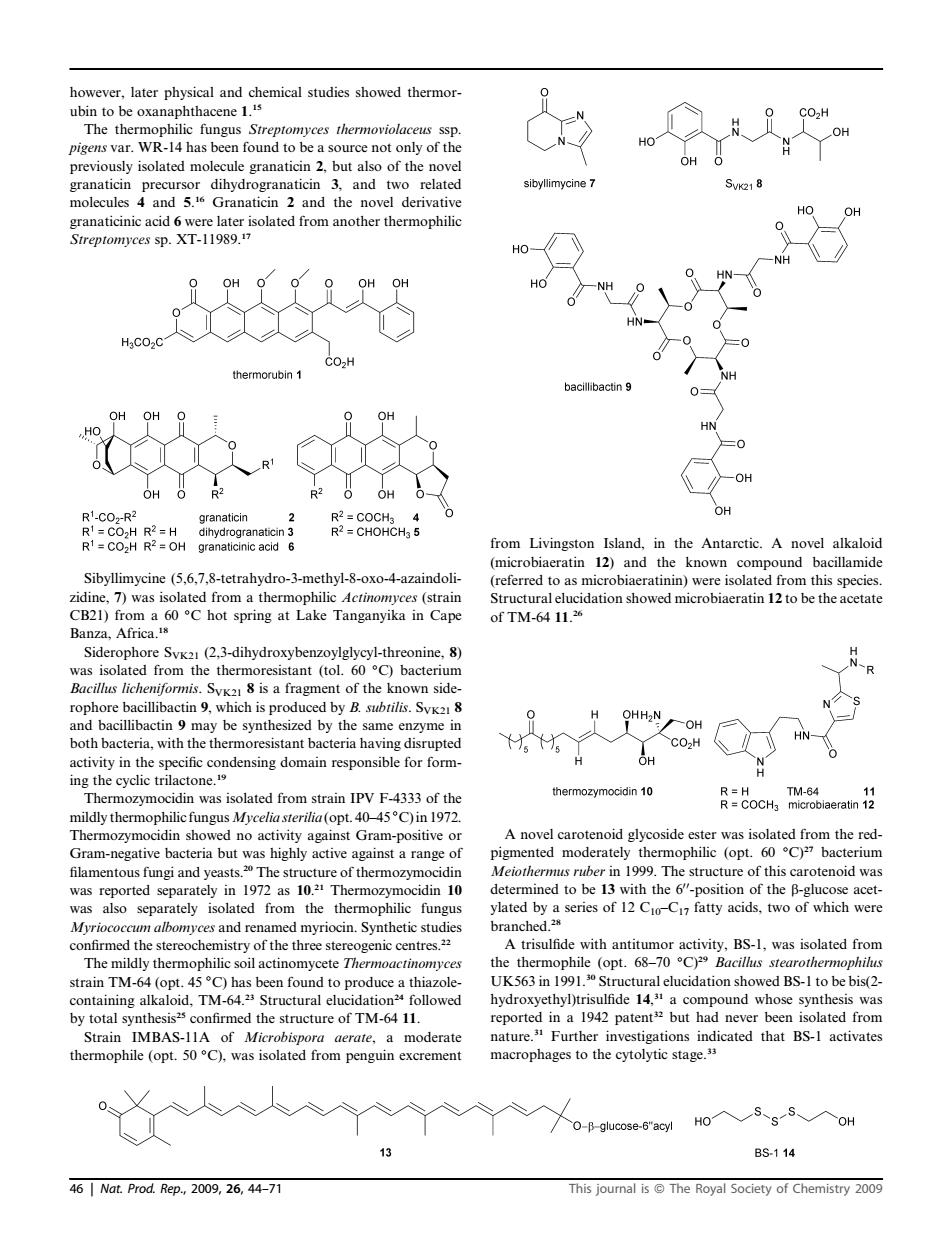
owever,later physical and chemical studies showed thermor The therm philic fungus Streptomyces thermoviolaceus ssp pigens var.W -14 has been found to be a source not only of the molecules 4 and 5.Granaticin 2 and the novel derivative HO- 一NH thermorubin 1 O OH 。o& OH RCHOHCH 5 from Livingston Island,in the Antarctic.A novel alkaloid (microbiaeratin 12)and the known compound bacillamide hilic Actinomyces (strain CB21)from a 60C hot spring at Lake Tanganyika in Cape of TM-64 11. .(2 3-dihydroxybenzovlglycyl-thre -R Bacillus licheniformis cn Is pro activity in the specific condensing domain responsible for form- Thermozymocidin was isolated from strain IPV F-4333 of the CoCH a mildly thermophilicfungus Myceliasterilia(opt.40-45C)in 1972 show no a s high against Gram-positive e filamentous fungi and yeasts.The structure of was reported separately in 1972 as 10. also sep tely confirmed the of the cn The AnindeihatnraeiiBSl.asobteao dly therm ophilic soil actinomyceteTh be bis(2 hydroxyethyl)trisulfide.a compound whose synthesis was by total synthesis confirmed the structure of TM-64 11. ature om 0 BS114 461 Nat.Prod..Rep,2009,26,44-77 This journal isThe Royal Society of Chemistry009 however, later physical and chemical studies showed thermorubin to be oxanaphthacene 1. 15 The thermophilic fungus Streptomyces thermoviolaceus ssp. pigens var. WR-14 has been found to be a source not only of the previously isolated molecule granaticin 2, but also of the novel granaticin precursor dihydrogranaticin 3, and two related molecules 4 and 5. 16 Granaticin 2 and the novel derivative granaticinic acid 6 were later isolated from another thermophilic Streptomyces sp. XT-11989.17 Sibyllimycine (5,6,7,8-tetrahydro-3-methyl-8-oxo-4-azaindolizidine, 7) was isolated from a thermophilic Actinomyces (strain CB21) from a 60 C hot spring at Lake Tanganyika in Cape Banza, Africa.18 Siderophore SVK21 (2,3-dihydroxybenzoylglycyl-threonine, 8) was isolated from the thermoresistant (tol. 60 C) bacterium Bacillus licheniformis. SVK21 8 is a fragment of the known siderophore bacillibactin 9, which is produced by B. subtilis. SVK21 8 and bacillibactin 9 may be synthesized by the same enzyme in both bacteria, with the thermoresistant bacteria having disrupted activity in the specific condensing domain responsible for forming the cyclic trilactone.19 Thermozymocidin was isolated from strain IPV F-4333 of the mildly thermophilic fungusMycelia sterilia (opt. 40–45 C) in 1972. Thermozymocidin showed no activity against Gram-positive or Gram-negative bacteria but was highly active against a range of filamentous fungi and yeasts.20 The structure of thermozymocidin was reported separately in 1972 as 10. 21 Thermozymocidin 10 was also separately isolated from the thermophilic fungus Myriococcum albomyces and renamed myriocin. Synthetic studies confirmed the stereochemistry of the three stereogenic centres.22 The mildly thermophilic soil actinomycete Thermoactinomyces strain TM-64 (opt. 45 C) has been found to produce a thiazolecontaining alkaloid, TM-64.23 Structural elucidation24 followed by total synthesis25 confirmed the structure of TM-64 11. Strain IMBAS-11A of Microbispora aerate, a moderate thermophile (opt. 50 C), was isolated from penguin excrement from Livingston Island, in the Antarctic. A novel alkaloid (microbiaeratin 12) and the known compound bacillamide (referred to as microbiaeratinin) were isolated from this species. Structural elucidation showed microbiaeratin 12 to be the acetate of TM-64 11. 26 A novel carotenoid glycoside ester was isolated from the redpigmented moderately thermophilic (opt. 60 C)27 bacterium Meiothermus ruber in 1999. The structure of this carotenoid was determined to be 13 with the 600-position of the b-glucose acetylated by a series of 12 C10–C17 fatty acids, two of which were branched.28 A trisulfide with antitumor activity, BS-1, was isolated from the thermophile (opt. 68–70 C)29 Bacillus stearothermophilus UK563 in 1991.30 Structural elucidation showed BS-1 to be bis(2- hydroxyethyl)trisulfide 14, 31 a compound whose synthesis was reported in a 1942 patent32 but had never been isolated from nature.31 Further investigations indicated that BS-1 activates macrophages to the cytolytic stage.33 46 | Nat. Prod. Rep., 2009, 26, 44–71 This journal is ª The Royal Society of Chemistry 2009