正在加载图片...
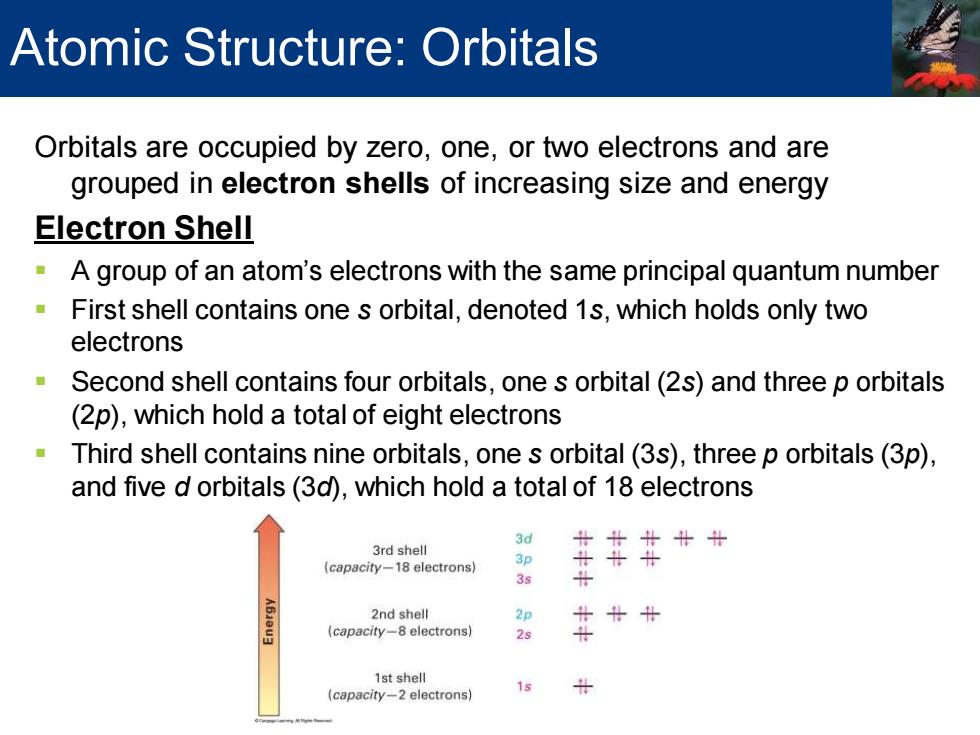
Atomic Structure:Orbitals Orbitals are occupied by zero,one,or two electrons and are grouped in electron shells of increasing size and energy Electron Shell A group of an atom's electrons with the same principal quantum number -First shell contains one s orbital,denoted 1s,which holds only two electrons Second shell contains four orbitals,one s orbital(2s)and three p orbitals (2p),which hold a total of eight electrons Third shell contains nine orbitals,one s orbital(3s),three p orbitals(3p), and five d orbitals(3d),which hold a total of 18 electrons 3d 3rd shell 北北计计计 (capacity-18 electrons) 3s 2nd shell 2p (capacity-8 electrons) 2s 1st shell 19 (capacity-2 electrons) Orbitals are occupied by zero, one, or two electrons and are grouped in electron shells of increasing size and energy Electron Shell ▪ A group of an atom’s electrons with the same principal quantum number ▪ First shell contains one s orbital, denoted 1s, which holds only two electrons ▪ Second shell contains four orbitals, one s orbital (2s) and three p orbitals (2p), which hold a total of eight electrons ▪ Third shell contains nine orbitals, one s orbital (3s), three p orbitals (3p), and five d orbitals (3d), which hold a total of 18 electrons Atomic Structure: Orbitals