正在加载图片...
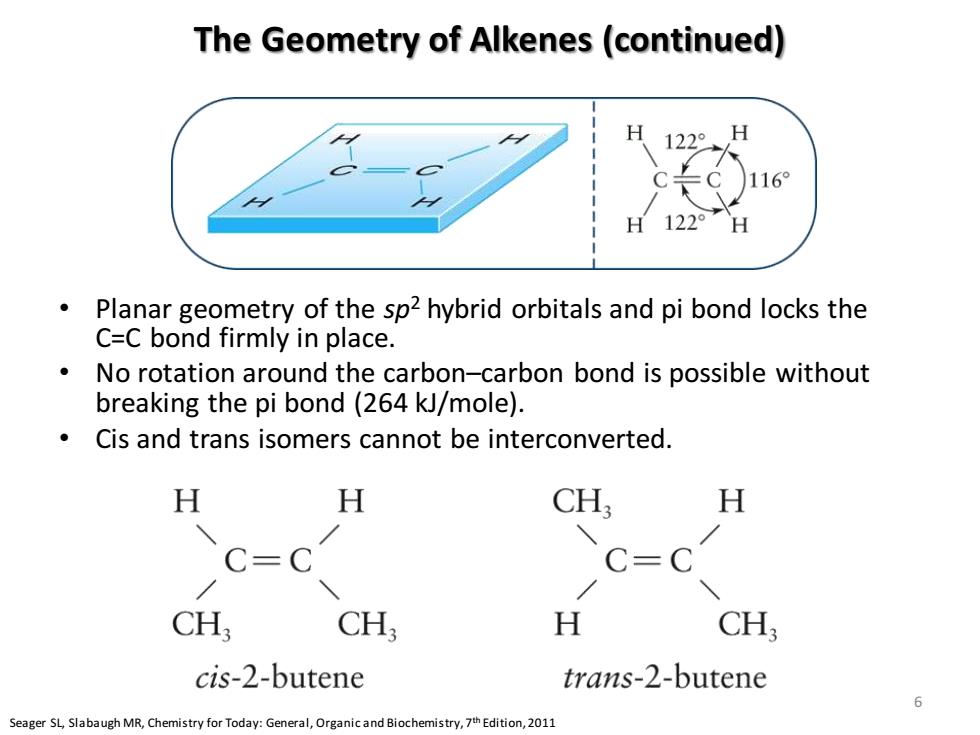
The Geometry of Alkenes(continued) 16 H 122° Planar geometry of the sp2 hybrid orbitals and pi bond locks the C=C bond firmly in place. No rotation around the carbon-carbon bond is possible without breaking the pi bond(264 kJ/mole). Cis and trans isomers cannot be interconverted. H H CH H C=C C=C CH; CH H CH, cis-2-butene trans-2-butene 6 Seager SLSlabaugh MR,Chemistry for Today:General,Organicand Biochemistry,Edition,2011 The Geometry of Alkenes (continued) • Planar geometry of the sp2 hybrid orbitals and pi bond locks the C=C bond firmly in place. • No rotation around the carbon–carbon bond is possible without breaking the pi bond (264 kJ/mole). • Cis and trans isomers cannot be interconverted. Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7th Edition, 2011 6