正在加载图片...
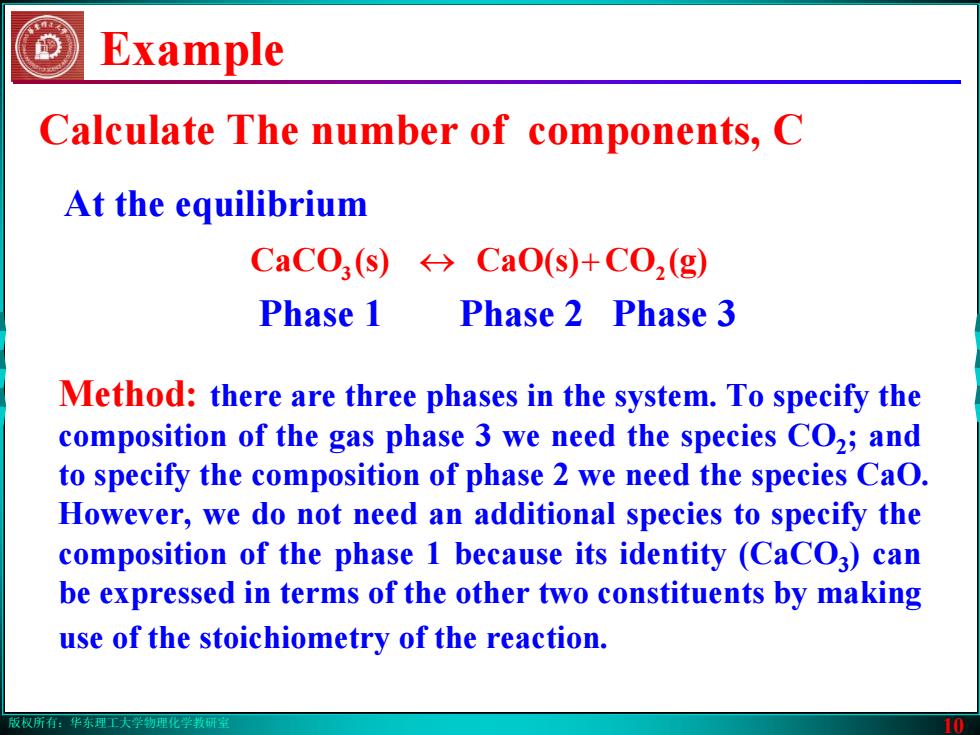
版权所有:华东理工大学物理化学教研室 10 Example Method: there are three phases in the system. To specify the composition of the gas phase 3 we need the species CO2; and to specify the composition of phase 2 we need the species CaO. However, we do not need an additional species to specify the composition of the phase 1 because its identity (CaCO3) can be expressed in terms of the other two constituents by making use of the stoichiometry of the reaction. At the equilibrium 3 ↔ CaO(s) (s)CaCO + 2 (g)CO Phase 1 Phase 2 Phase 3 Calculate The number of components, C版权所有:华东理工大学物理化学教研室 10 Example Method: there are three phases in the system. To specify the composition of the gas phase 3 we need the species CO2; and to specify the composition of phase 2 we need the species CaO. However, we do not need an additional species to specify the composition of the phase 1 because its identity (CaCO3) can be expressed in terms of the other two constituents by making use of the stoichiometry of the reaction. At the equilibrium 3 ↔ CaO(s) (s)CaCO + 2 (g)CO Phase 1 Phase 2 Phase 3 Calculate The number of components, C