正在加载图片...
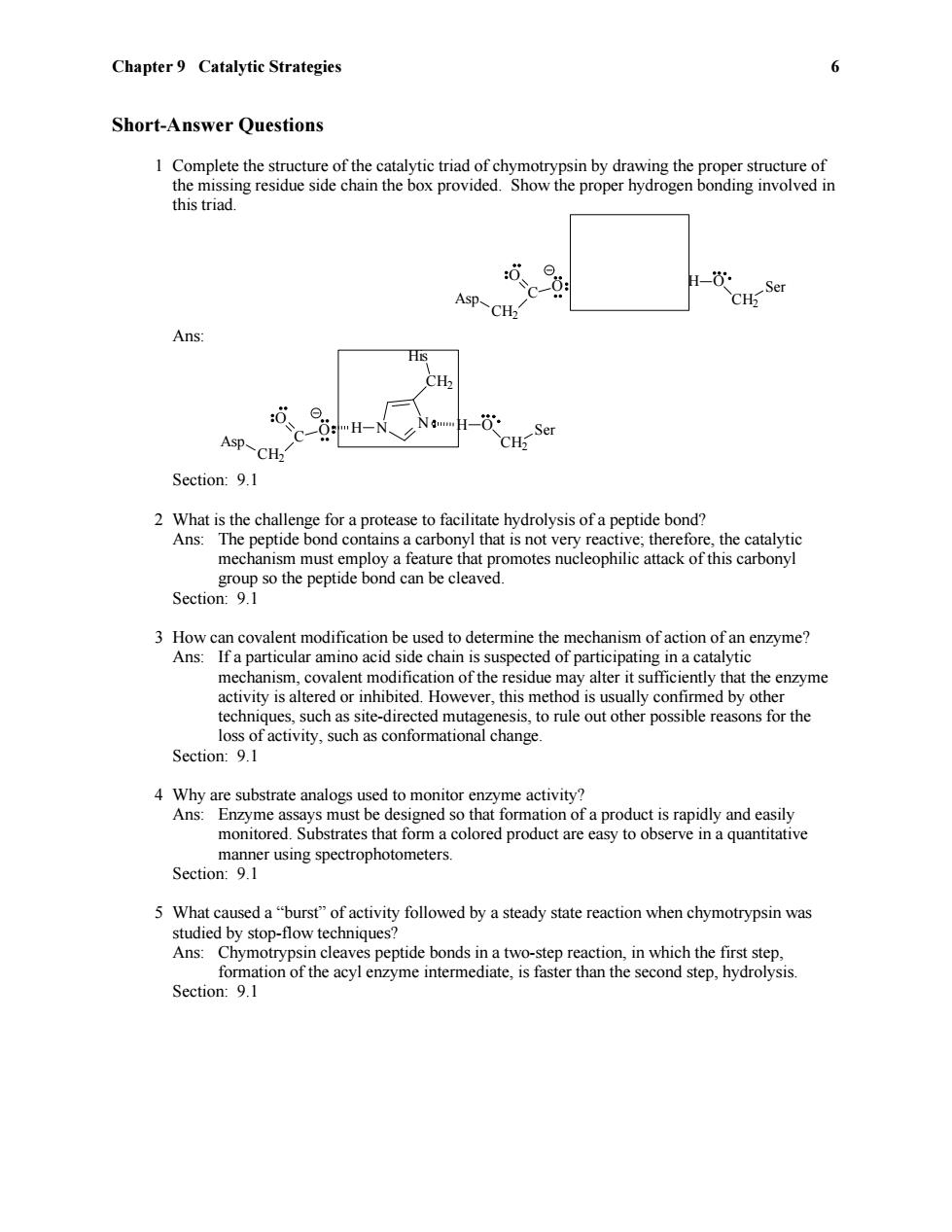
Chapter 9 Catalytic Strategies 6 Short-Answer Questions 1 Complete the structure of the catalytic triad of chymotrypsin by drawing the proper structure of the missing residue side chain the box provided.Show the proper hydrogen bonding involved in this triad. Asp-CH2 CH-Ser Ans: CH Asp-CH2 CH;Ser Section:9.1 2 What is the challenge for a protease to facilitate hydrolysis of a peptide bond? Ans:The peptide bond contains a carbonyl that is not very reactive;therefore,the catalytic mechanism must employ a feature that promotes nucleophilic attack of this carbonyl group so the peptide bond can be cleaved. Section:9.1 3 How can covalent modification be used to determine the mechanism of action of an enzyme? Ans:If a particular amino acid side chain is suspected of participating in a catalytic mechanism,covalent modification of the residue may alter it sufficiently that the enzyme activity is altered or inhibited.However,this method is usually confirmed by other techniques,such as site-directed mutagenesis,to rule out other possible reasons for the loss of activity,such as conformational change. Section:9.1 4 Why are substrate analogs used to monitor enzyme activity? Ans:Enzyme assays must be designed so that formation of a product is rapidly and easily monitored.Substrates that form a colored product are easy to observe in a quantitative manner using spectrophotometers. Section:9.1 5 What caused a"burst"of activity followed by a steady state reaction when chymotrypsin was studied by stop-flow techniques? Ans:Chymotrypsin cleaves peptide bonds in a two-step reaction,in which the first step, formation of the acyl enzyme intermediate,is faster than the second step,hydrolysis Section:9.1Chapter 9 Catalytic Strategies 6 Short-Answer Questions 1 Complete the structure of the catalytic triad of chymotrypsin by drawing the proper structure of the missing residue side chain the box provided. Show the proper hydrogen bonding involved in this triad. H O CH2 Ser C O O CH2 Asp Ans: H O CH2 H N Ser N CH2 His C O O CH2 Asp Section: 9.1 2 What is the challenge for a protease to facilitate hydrolysis of a peptide bond? Ans: The peptide bond contains a carbonyl that is not very reactive; therefore, the catalytic mechanism must employ a feature that promotes nucleophilic attack of this carbonyl group so the peptide bond can be cleaved. Section: 9.1 3 How can covalent modification be used to determine the mechanism of action of an enzyme? Ans: If a particular amino acid side chain is suspected of participating in a catalytic mechanism, covalent modification of the residue may alter it sufficiently that the enzyme activity is altered or inhibited. However, this method is usually confirmed by other techniques, such as site-directed mutagenesis, to rule out other possible reasons for the loss of activity, such as conformational change. Section: 9.1 4 Why are substrate analogs used to monitor enzyme activity? Ans: Enzyme assays must be designed so that formation of a product is rapidly and easily monitored. Substrates that form a colored product are easy to observe in a quantitative manner using spectrophotometers. Section: 9.1 5 What caused a “burst” of activity followed by a steady state reaction when chymotrypsin was studied by stop-flow techniques? Ans: Chymotrypsin cleaves peptide bonds in a two-step reaction, in which the first step, formation of the acyl enzyme intermediate, is faster than the second step, hydrolysis. Section: 9.1