正在加载图片...
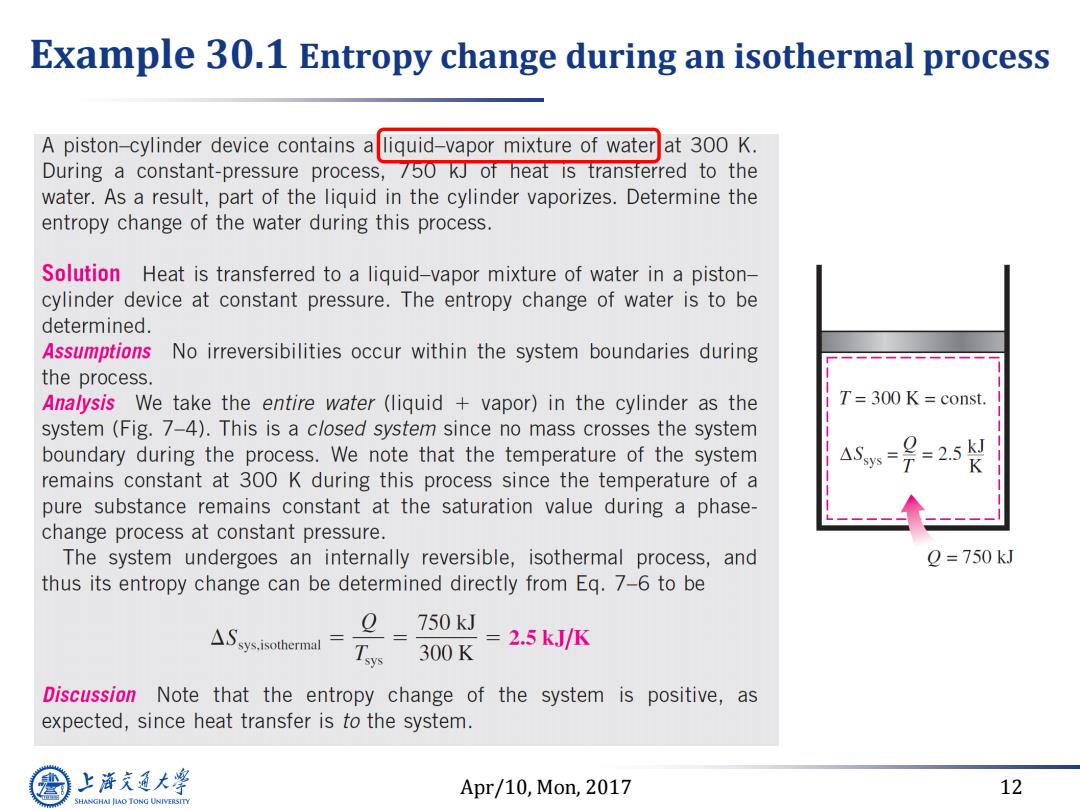
Example 30.1 Entropy change during an isothermal process A piston-cylinder device contains aliquid-vapor mixture of water at 300 K. During a constant-pressure process,750 kJ of heat is transterred to the water.As a result,part of the liquid in the cylinder vaporizes.Determine the entropy change of the water during this process. Solution Heat is transferred to a liquid-vapor mixture of water in a piston- cylinder device at constant pressure.The entropy change of water is to be determined. Assumptions No irreversibilities occur within the system boundaries during the process. Analysis We take the entire water (liquid vapor)in the cylinder as the T=300 K const. system (Fig.7-4).This is a closed system since no mass crosses the system boundary during the process.We note that the temperature of the system ASsys 号=25 remains constant at 300 K during this process since the temperature of a pure substance remains constant at the saturation value during a phase- change process at constant pressure. The system undergoes an internally reversible,isothermal process,and 2=750kJ thus its entropy change can be determined directly from Eq.7-6 to be 0 △Ssys.isothermal= Tsys 750kJ=2.5kJ/K 300K Discussion Note that the entropy change of the system is positive,as expected,since heat transfer is to the system. 上降文通大学 Apr/10,Mon,2017 12 SHANGHAI JIAO TONG UNIVERSITYApr/10, Mon, 2017 12 Example 30.1 Entropy change during an isothermal process