正在加载图片...
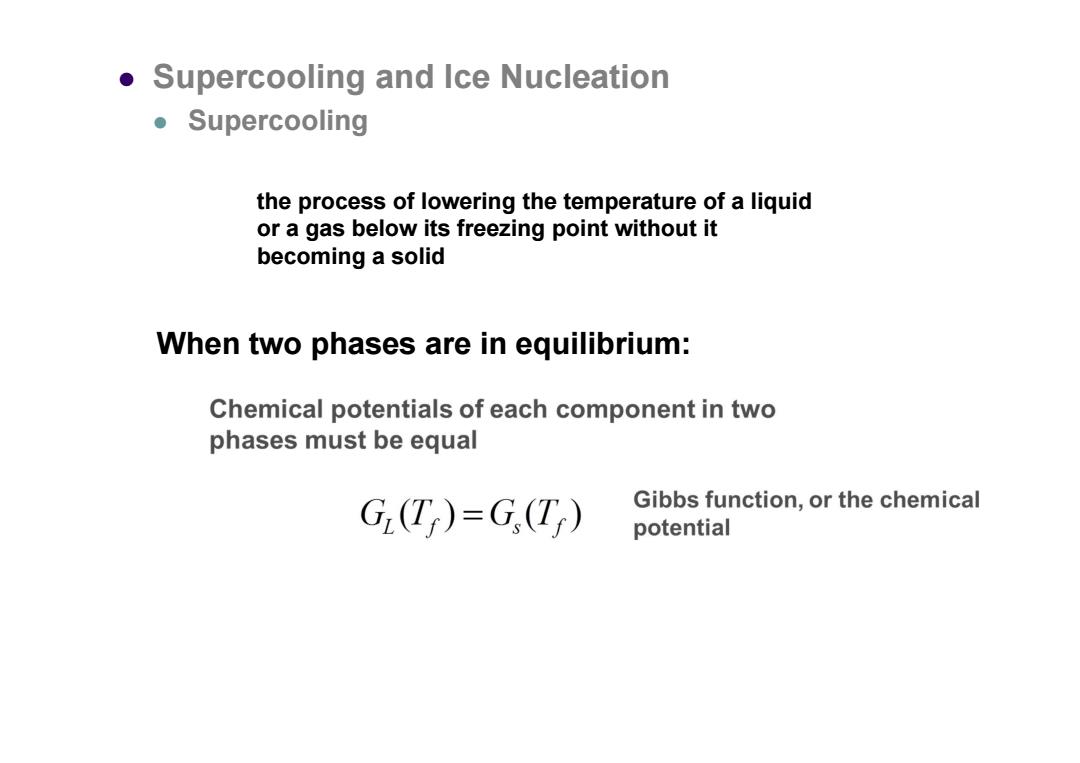
Supercooling and Ice Nucleation ●Supercooling the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid When two phases are in equilibrium: Chemical potentials of each component in two phases must be equal G(T)=G(T) Gibbs function,or the chemical potentialthe process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid Supercooling and Ice Nucleation Supercooling When two phases are in equilibrium: