正在加载图片...
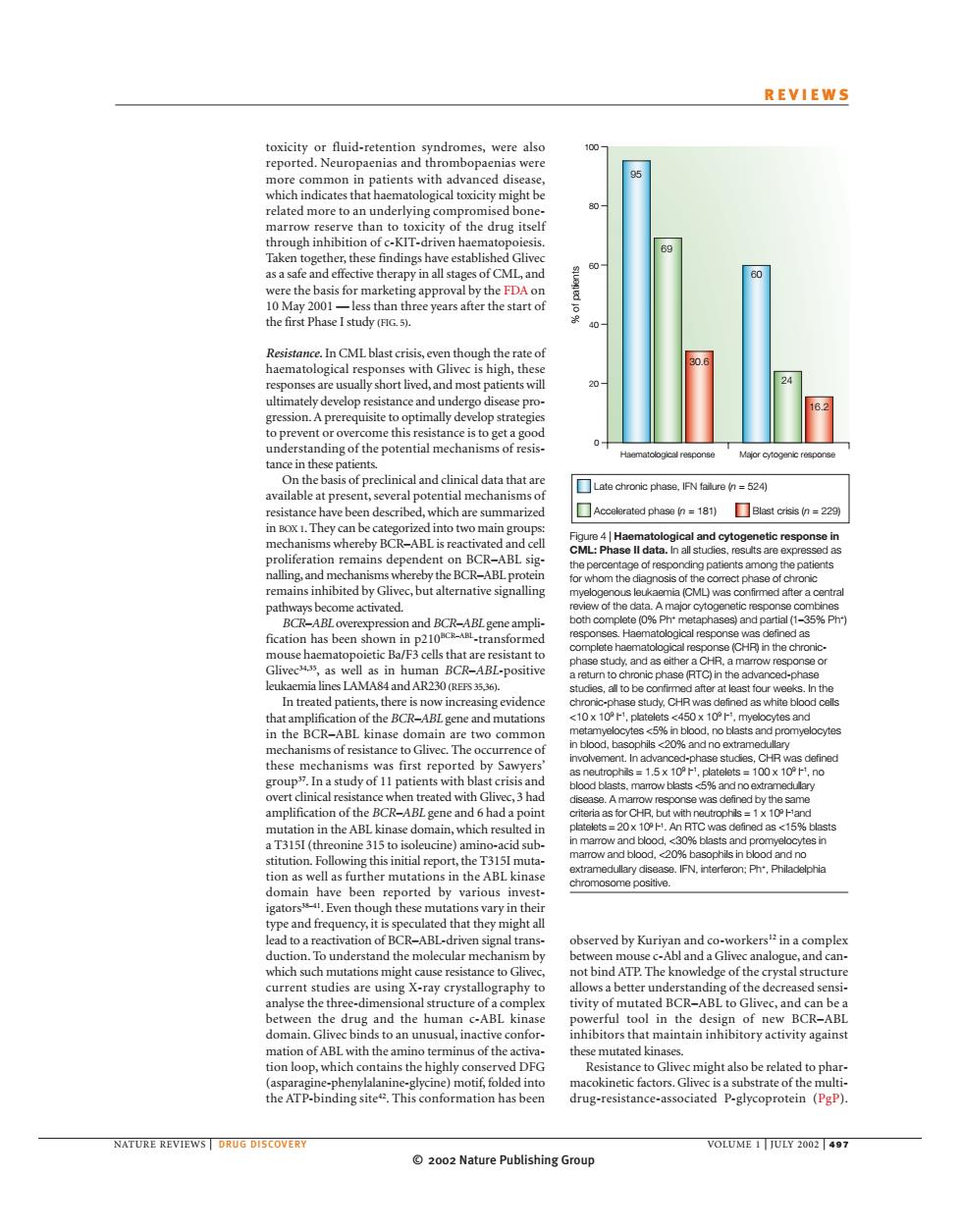
REVIEWS the drug itse thefirst Phase I study). ps虹 小吧 nding of the 52 been descrbed,which are summ A erated phese n=181)Blast crisis n be bc R-ABL but alternative sig 语nh d natient c5 20成0。 rence mplit sI.gene an An BT 15 51( nine ion well as further mutat in t gato these m vary in bserved by ATP.The know ge of the crystal structur ructure vity of mut ABL to Gliv nacti ionioopwhiaht ins the highly sistance to gliv might also be related to phar the ATP-bindingite This been NATURE REVIEWS OLUME 2002 Nature Publishing Group© 2002 Nature Publishing Group NATURE REVIEWS | DRUG DISCOVERY VOLUME 1 | JULY 2002 | 497 REVIEWS observed by Kuriyan and co-workers12 in a complex between mouse c-Abl and a Glivec analogue, and cannot bind ATP. The knowledge of the crystal structure allows a better understanding of the decreased sensitivity of mutated BCR–ABL to Glivec, and can be a powerful tool in the design of new BCR–ABL inhibitors that maintain inhibitory activity against these mutated kinases. Resistance to Glivec might also be related to pharmacokinetic factors. Glivec is a substrate of the multidrug-resistance-associated P-glycoprotein (PgP). toxicity or fluid-retention syndromes, were also reported. Neuropaenias and thrombopaenias were more common in patients with advanced disease, which indicates that haematological toxicity might be related more to an underlying compromised bonemarrow reserve than to toxicity of the drug itself through inhibition of c-KIT-driven haematopoiesis. Taken together, these findings have established Glivec as a safe and effective therapy in all stages of CML, and were the basis for marketing approval by the FDA on 10 May 2001 — less than three years after the start of the first Phase I study (FIG. 5). Resistance. In CML blast crisis, even though the rate of haematological responses with Glivec is high, these responses are usually short lived, and most patients will ultimately develop resistance and undergo disease progression. A prerequisite to optimally develop strategies to prevent or overcome this resistance is to get a good understanding of the potential mechanisms of resistance in these patients. On the basis of preclinical and clinical data that are available at present, several potential mechanisms of resistance have been described, which are summarized in BOX 1. They can be categorized into two main groups: mechanisms whereby BCR–ABL is reactivated and cell proliferation remains dependent on BCR–ABL signalling, and mechanisms whereby the BCR–ABL protein remains inhibited by Glivec, but alternative signalling pathways become activated. BCR–ABL overexpression and BCR–ABL gene amplification has been shown in p210BCR–ABL-transformed mouse haematopoietic Ba/F3 cells that are resistant to Glivec34,35, as well as in human BCR–ABL-positive leukaemia lines LAMA84 and AR230 (REFS 35,36). In treated patients, there is now increasing evidence that amplification of the BCR–ABL gene and mutations in the BCR–ABL kinase domain are two common mechanisms of resistance to Glivec. The occurrence of these mechanisms was first reported by Sawyers’ group37. In a study of 11 patients with blast crisis and overt clinical resistance when treated with Glivec, 3 had amplification of the BCR–ABL gene and 6 had a point mutation in the ABL kinase domain, which resulted in a T315I (threonine 315 to isoleucine) amino-acid substitution. Following this initial report, the T315I mutation as well as further mutations in the ABL kinase domain have been reported by various investigators38–41. Even though these mutations vary in their type and frequency, it is speculated that they might all lead to a reactivation of BCR–ABL-driven signal transduction. To understand the molecular mechanism by which such mutations might cause resistance to Glivec, current studies are using X-ray crystallography to analyse the three-dimensional structure of a complex between the drug and the human c-ABL kinase domain. Glivec binds to an unusual, inactive conformation of ABL with the amino terminus of the activation loop, which contains the highly conserved DFG (asparagine-phenylalanine-glycine) motif, folded into the ATP-binding site42. This conformation has been Haematological response Major cytogenic response % of patients 100 80 60 40 20 0 Late chronic phase, IFN failure (n = 524) Accelerated phase (n = 181) Blast crisis (n = 229) 95 69 30.6 60 24 16.2 Figure 4 | Haematological and cytogenetic response in CML: Phase II data. In all studies, results are expressed as the percentage of responding patients among the patients for whom the diagnosis of the correct phase of chronic myelogenous leukaemia (CML) was confirmed after a central review of the data. A major cytogenetic response combines both complete (0% Ph+ metaphases) and partial (1–35% Ph+) responses. Haematological response was defined as complete haematological response (CHR) in the chronicphase study, and as either a CHR, a marrow response or a return to chronic phase (RTC) in the advanced-phase studies, all to be confirmed after at least four weeks. In the chronic-phase study, CHR was defined as white blood cells <10 x 109 l –1, platelets <450 x 109 l –1, myelocytes and metamyelocytes <5% in blood, no blasts and promyelocytes in blood, basophils <20% and no extramedullary involvement. In advanced-phase studies, CHR was defined as neutrophils = 1.5 x 109 l –1, platelets = 100 x 109 l –1, no blood blasts, marrow blasts <5% and no extramedullary disease. A marrow response was defined by the same criteria as for CHR, but with neutrophils = 1 x 109 l –1 and platelets = 20 x 109 l –1 . An RTC was defined as <15% blasts in marrow and blood, <30% blasts and promyelocytes in marrow and blood, <20% basophils in blood and no extramedullary disease. IFN, interferon; Ph+, Philadelphia chromosome positive