正在加载图片...
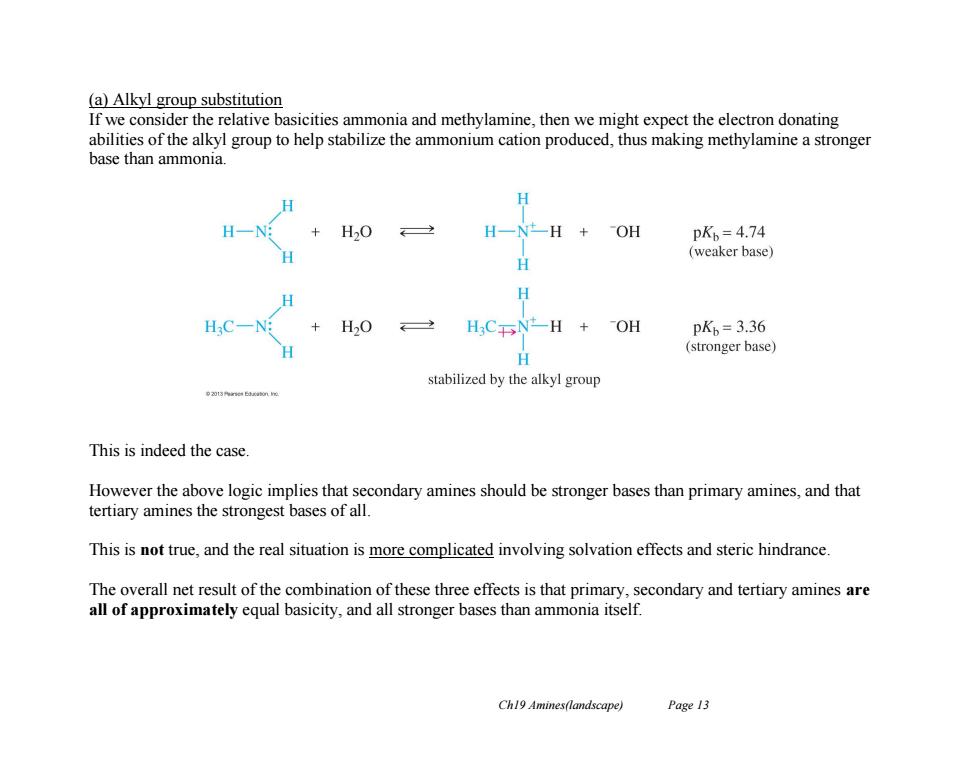
(a)Alkyl group substitution If we consider the relative basicities ammonia and methylamine,then we might expect the electron donating abilities of the alkyl group to help stabilize the ammonium cation produced,thus making methylamine a stronger base than ammonia. H H-N H一N一H+OH pKb=4.74 (weaker base) H HC一N +H20 2 H3CNH +OH pK=3.36 (stronger base) 日 stabilized by the alkyl group This is indeed the case. However the above logic implies that secondary amines should be stronger bases than primary amines,and that tertiary amines the strongest bases of all. This is not true,and the real situation is more complicated involving solvation effects and steric hindrance. The overall net result of the combination of these three effects is that primary,secondary and tertiary amines are all of approximately equal basicity,and all stronger bases than ammonia itself. Ch19 Amines(landscape) Page 13Ch19 Amines(landscape) Page 13 (a) Alkyl group substitution If we consider the relative basicities ammonia and methylamine, then we might expect the electron donating abilities of the alkyl group to help stabilize the ammonium cation produced, thus making methylamine a stronger base than ammonia. This is indeed the case. However the above logic implies that secondary amines should be stronger bases than primary amines, and that tertiary amines the strongest bases of all. This is not true, and the real situation is more complicated involving solvation effects and steric hindrance. The overall net result of the combination of these three effects is that primary, secondary and tertiary amines are all of approximately equal basicity, and all stronger bases than ammonia itself