正在加载图片...
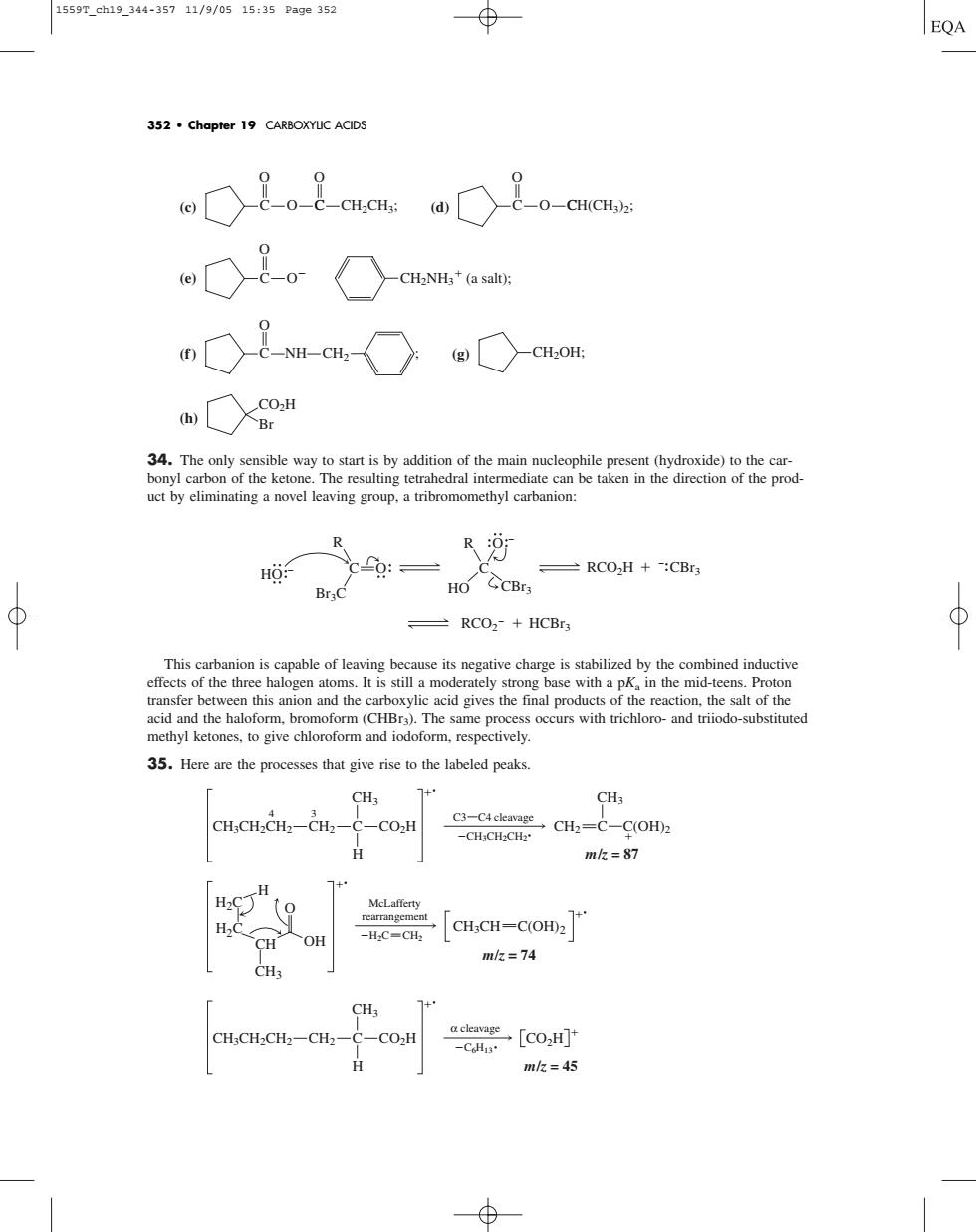
15597.eh19_344-35711/9/0515:35Page352 EQA 352.chapter 19 CARBOXYUC ACIDS oO是o是aawO是。eas oa 34.The only sensible way to start is by addition of the main nucleophile present (hydroxide)to the car- bonyl carbon of the ketone.The resulting tetrahedral intermediate can be taken in the direction of the prod- ct by eliminating a novel leaving group,a tribromomethyl carbanion R R: -RCO2H+:CBr Br:C HO CCBrs 一RCO2+HCBS 35.Here are the processes that give rise to the labeled peaks. CH3 CHCH-CH2-CH2-C-CO.H H mk=87 H,CC人oH 「cH,cH=CoH2 mk=74 CH;CH-CH2-CH2-C-CO:H [CO,H] H mk=45(c) (d) (e) (f) (g) (h) 34. The only sensible way to start is by addition of the main nucleophile present (hydroxide) to the carbonyl carbon of the ketone. The resulting tetrahedral intermediate can be taken in the direction of the product by eliminating a novel leaving group, a tribromomethyl carbanion: This carbanion is capable of leaving because its negative charge is stabilized by the combined inductive effects of the three halogen atoms. It is still a moderately strong base with a pKa in the mid-teens. Proton transfer between this anion and the carboxylic acid gives the final products of the reaction, the salt of the acid and the haloform, bromoform (CHBr3). The same process occurs with trichloro- and triiodo-substituted methyl ketones, to give chloroform and iodoform, respectively. 35. Here are the processes that give rise to the labeled peaks. α cleavage C6H13 CO2H m/z = 45 CH3CH2CH2 CH2 C CO2H H CH3 O H H2C H2C CH3 CH OH McLafferty rearrangement H2C CH2 CH3CH C(OH)2 m/z = 74 C3 C4 cleavage CH3CH2CH2 CH3CH2CH2 CH2 C CO2H H CH3 4 3 m/z = 87 CH3 CH2 C C(OH)2 C O Br3C R HO C O CBr3 RCO2H HO R CBr3 RCO2 HCBr3 CO2H Br C NH CH2OH; O CH2 ; C O O CH2NH3 (a salt); C C OH(CH3)2; O C C O CH2CH3; O O 352 • Chapter 19 CARBOXYLIC ACIDS 1559T_ch19_344-357 11/9/05 15:35 Page 352�����������������