正在加载图片...
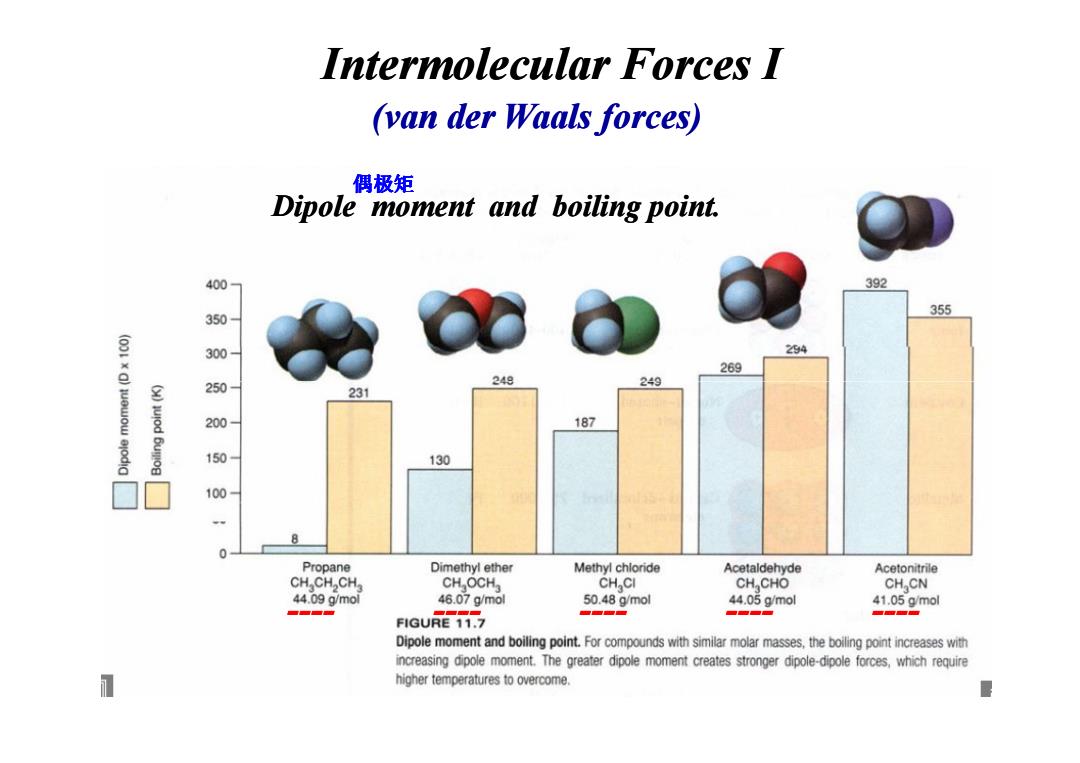
Intermolecular Forces I (van der Waals forces) 偶极矩 Dipole moment and boiling point. 400 392 355 350 豆 300 294 269 250 249 249 231 200 187 150 130 100 8 0 Propane Dimethyl ether Methyl chloride Acetaldehyde Acetonitrile CH CH CH CH OCH CH CI CH CHO CH CN 44.09g/mol 46.07g/mal 50.48g/mol 44.05g/mol 41.05g/mol 。 。雪学■ 。海。 FIGURE 11.7 Dipole moment and boiling point.For compounds with similar molar masses,the boiling point increases with increasing dipole moment.The greater dipole moment creates stronger dipole-dipole forces,which require higher temperatures to overcome.Intermolecular Forces I (van der Waals forces) Dipole moment and boiling point. 偶极矩 ---- ---- ---- ---- ----