正在加载图片...
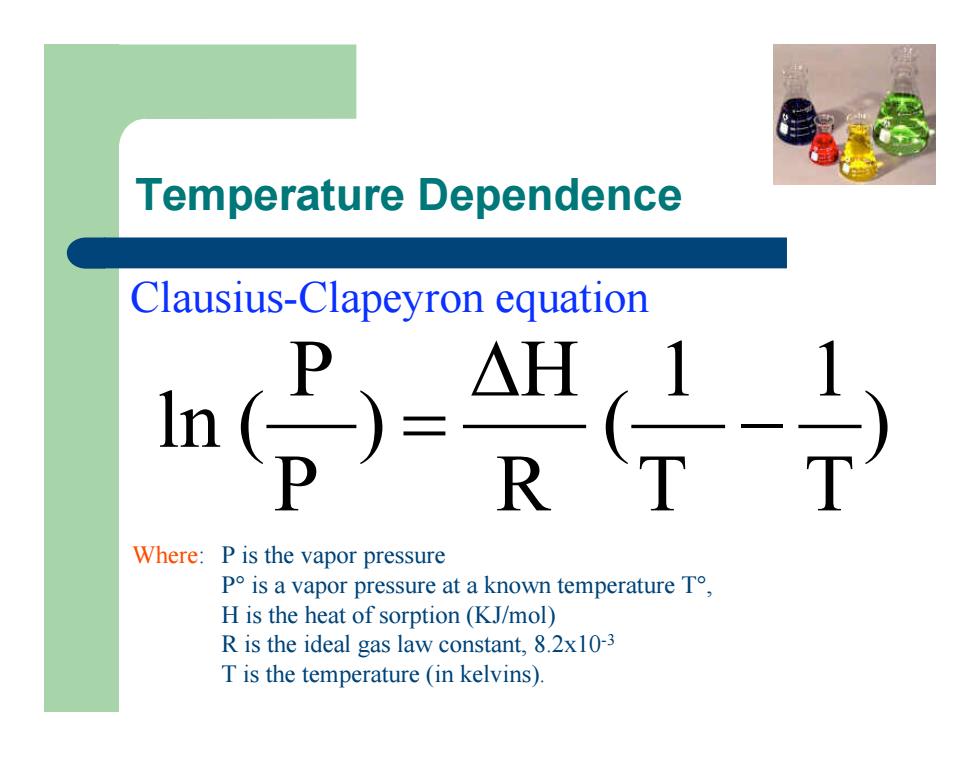
Temperature Dependence ) T 1 T 1 ( R H ) P P ln ( - D = Clausius-Clapeyron equation Where: P is the vapor pressure P° is a vapor pressure at a known temperature T°, H is the heat of sorption (KJ/mol) R is the ideal gas law constant, 8.2x10-3 T is the temperature (in kelvins).Temperature Dependence ) T 1 T 1 ( R H ) P P ln ( - D = Clausius-Clapeyron equation Where: P is the vapor pressure P° is a vapor pressure at a known temperature T°, H is the heat of sorption (KJ/mol) R is the ideal gas law constant, 8.2x10-3 T is the temperature (in kelvins)