正在加载图片...
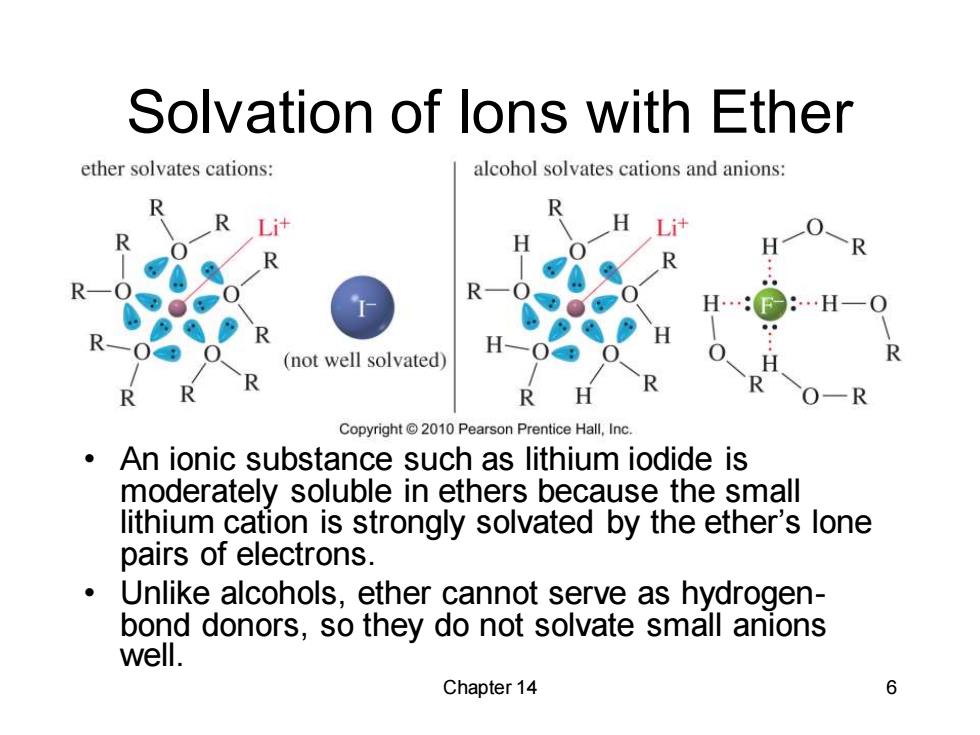
Solvation of lons with Ether ether solvates cations: alcohol solvates cations and anions: R (not well solvated) R O-R Copyright2010 Pearson Prentice Hall,Inc. An ionic substance such as lithium iodide is moderately soluble in ethers because the small lithium cation is strongly solvated by the ether's lone pairs of electrons. Unlike alcohols,ether cannot serve as hydrogen- bond donors,so they do not solvate small anions well. Chapter 14 6 Chapter 14 6 Solvation of Ions with Ether • An ionic substance such as lithium iodide is moderately soluble in ethers because the small lithium cation is strongly solvated by the ether’s lone pairs of electrons. • Unlike alcohols, ether cannot serve as hydrogenbond donors, so they do not solvate small anions well