正在加载图片...
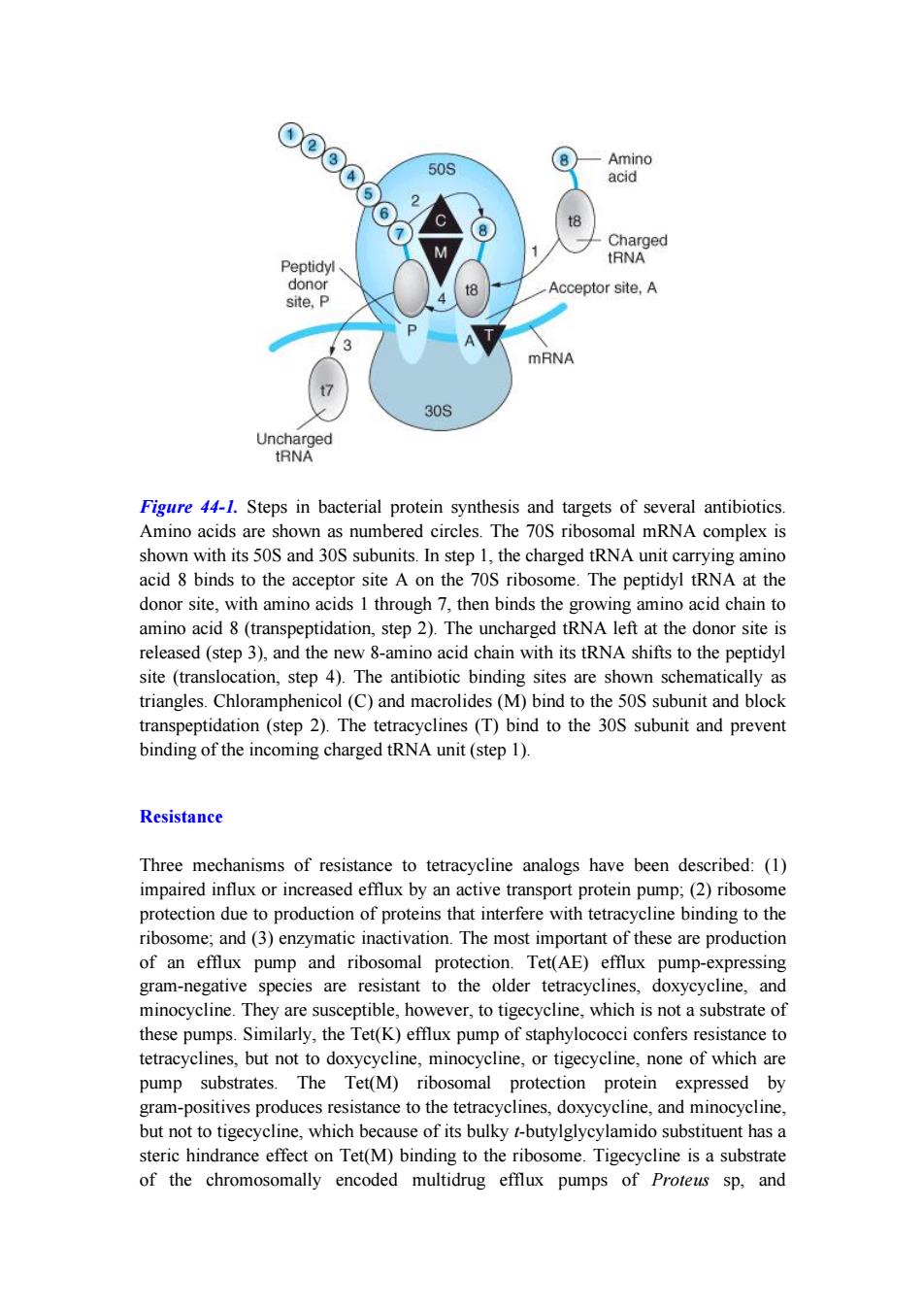
⊙@⊙ 8 Amino 50S acid (6 2 t8 Charged Peptidyl tRNA donor Acceptor site,A site,P 3 mRNA 30S Uncharged tRNA Figure 44-1.Steps in bacterial protein synthesis and targets of several antibiotics. Amino acids are shown as numbered circles.The 70S ribosomal mRNA complex is shown with its 50S and 30S subunits.In step 1,the charged tRNA unit carrying amino acid 8 binds to the acceptor site A on the 70S ribosome.The peptidyl tRNA at the donor site,with amino acids 1 through 7,then binds the growing amino acid chain to amino acid 8(transpeptidation,step 2).The uncharged tRNA left at the donor site is released(step 3),and the new 8-amino acid chain with its tRNA shifts to the peptidyl site (translocation,step 4).The antibiotic binding sites are shown schematically as triangles.Chloramphenicol(C)and macrolides(M)bind to the 50S subunit and block transpeptidation(step 2).The tetracyclines(T)bind to the 30S subunit and prevent binding of the incoming charged tRNA unit(step 1). Resistance Three mechanisms of resistance to tetracycline analogs have been described:(1) impaired influx or increased efflux by an active transport protein pump;(2)ribosome protection due to production of proteins that interfere with tetracycline binding to the ribosome;and(3)enzymatic inactivation.The most important of these are production of an efflux pump and ribosomal protection.Tet(AE)efflux pump-expressing gram-negative species are resistant to the older tetracyclines,doxycycline,and minocycline.They are susceptible,however,to tigecycline,which is not a substrate of these pumps.Similarly,the Tet(K)efflux pump of staphylococci confers resistance to tetracyclines,but not to doxycycline,minocycline,or tigecycline,none of which are pump substrates.The Tet(M)ribosomal protection protein expressed by gram-positives produces resistance to the tetracyclines,doxycycline,and minocycline, but not to tigecycline,which because of its bulky t-butylglycylamido substituent has a steric hindrance effect on Tet(M)binding to the ribosome.Tigecycline is a substrate of the chromosomally encoded multidrug efflux pumps of Proteus sp,andFigure 44-1. Steps in bacterial protein synthesis and targets of several antibiotics. Amino acids are shown as numbered circles. The 70S ribosomal mRNA complex is shown with its 50S and 30S subunits. In step 1, the charged tRNA unit carrying amino acid 8 binds to the acceptor site A on the 70S ribosome. The peptidyl tRNA at the donor site, with amino acids 1 through 7, then binds the growing amino acid chain to amino acid 8 (transpeptidation, step 2). The uncharged tRNA left at the donor site is released (step 3), and the new 8-amino acid chain with its tRNA shifts to the peptidyl site (translocation, step 4). The antibiotic binding sites are shown schematically as triangles. Chloramphenicol (C) and macrolides (M) bind to the 50S subunit and block transpeptidation (step 2). The tetracyclines (T) bind to the 30S subunit and prevent binding of the incoming charged tRNA unit (step 1). Resistance Three mechanisms of resistance to tetracycline analogs have been described: (1) impaired influx or increased efflux by an active transport protein pump; (2) ribosome protection due to production of proteins that interfere with tetracycline binding to the ribosome; and (3) enzymatic inactivation. The most important of these are production of an efflux pump and ribosomal protection. Tet(AE) efflux pump-expressing gram-negative species are resistant to the older tetracyclines, doxycycline, and minocycline. They are susceptible, however, to tigecycline, which is not a substrate of these pumps. Similarly, the Tet(K) efflux pump of staphylococci confers resistance to tetracyclines, but not to doxycycline, minocycline, or tigecycline, none of which are pump substrates. The Tet(M) ribosomal protection protein expressed by gram-positives produces resistance to the tetracyclines, doxycycline, and minocycline, but not to tigecycline, which because of its bulky t-butylglycylamido substituent has a steric hindrance effect on Tet(M) binding to the ribosome. Tigecycline is a substrate of the chromosomally encoded multidrug efflux pumps of Proteus sp, and