正在加载图片...
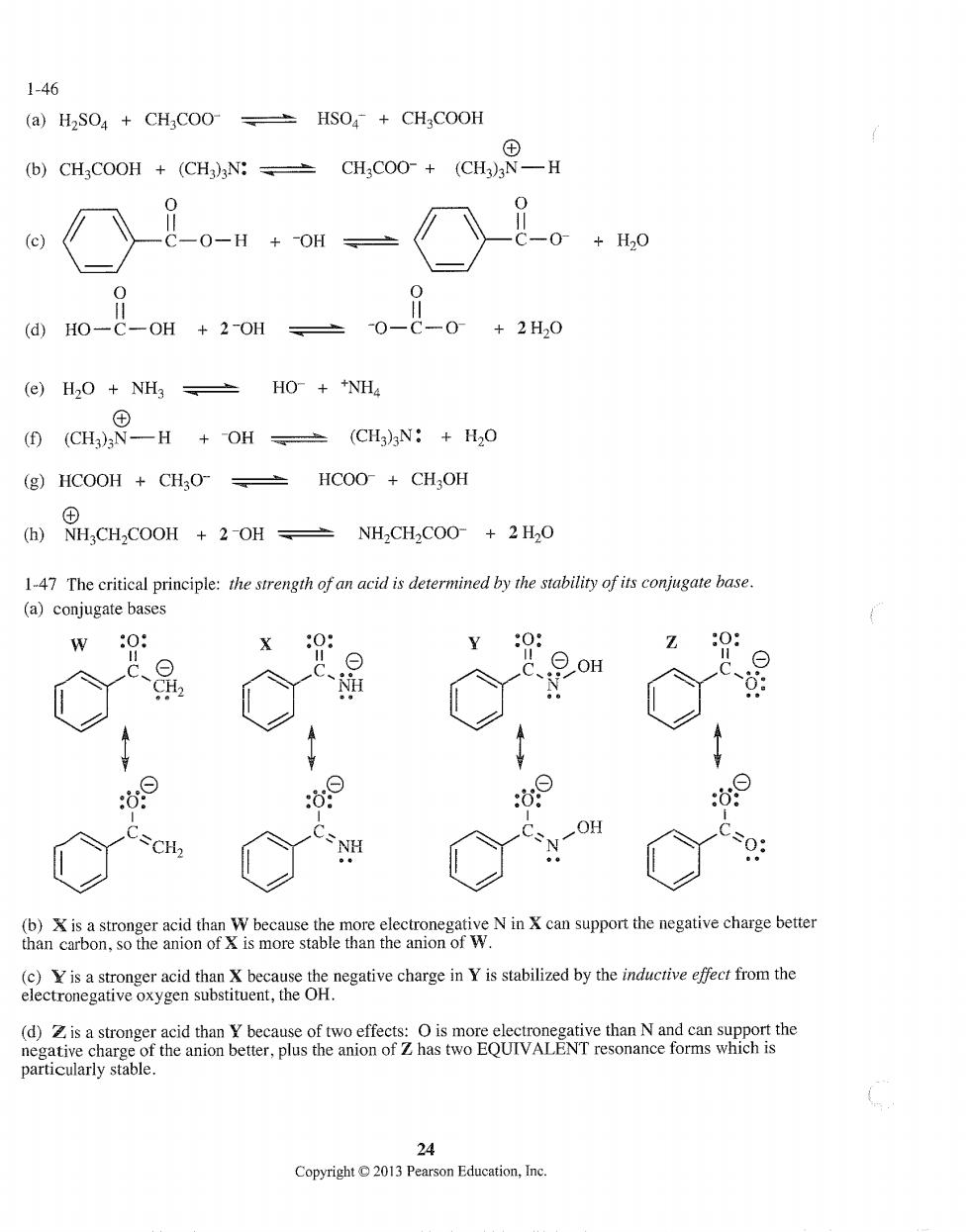
1-46 (a)H2SO4+CH COO HSO+CHCOOH (CHCOOH+CHCH.COO+(C -0-H+0 +H,0 0 @H0-C-0H+2-0H=0-C-0+2H,0 (e)H,0+NH3±H0+NH4 C9-H+OH、CHN:+0 (g)HCOOH CHO-HCOO CH,OH (h)NHCH2COOH +2-OH NH2CH2COO-2H2O 1-47 The critical principle:the strength of an acid is determined by the stability of its conjugate base (a)conjugate bases w0: 0: Y0: Z0: oH :⊙ 次⊙ OH CH (b)Xis astronger acid than W because the more electronegative Nin can suppor the negative charge better than carbon,so the anion of is more stable than the anion of W. (c)Yis a stronger acid than X because the negative charge in Y is stabilized by the inducrive effect from the electronegative oxygen substituent,the OH. (d)is a stron anion b particularly stable. 24 Copyright 2013 Pearson Education,Inc