正在加载图片...
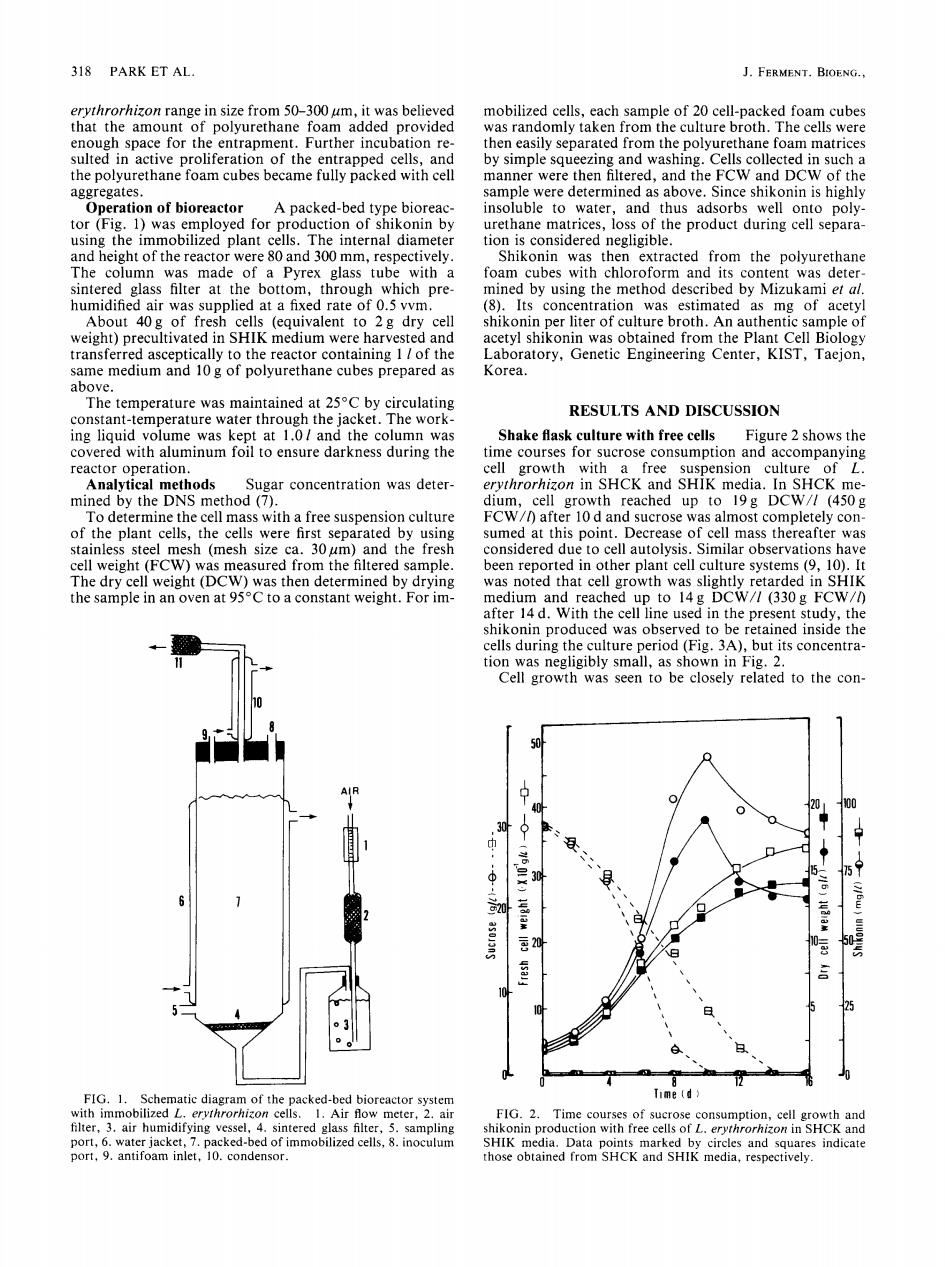
318 PARK ET AL J.FERMENT.BIOENG., erythrorhizon range in size from 50-300 um,it was believed mobilized cells,each sample of 20 cell-packed foam cubes that the amount of polyurethane foam added provided was randomly taken from the culture broth.The cells were enough space for the entrapment.Further incubation re- then easily separated from the polyurethane foam matrices sulted in active proliferation of the entrapped cells,and by simple squeezing and washing.Cells collected in such a the polyurethane foam cubes became fully packed with cell manner were then filtered,and the FCW and DCW of the aggregates. sample were determined as above.Since shikonin is highly Operation of bioreactor A packed-bed type bioreac- insoluble to water,and thus adsorbs well onto poly- tor(Fig.1)was employed for production of shikonin by urethane matrices,loss of the product during cell separa- using the immobilized plant cells.The internal diameter tion is considered negligible. and height of the reactor were 80 and 300 mm,respectively. Shikonin was then extracted from the polyurethane The column was made of a Pyrex glass tube with a foam cubes with chloroform and its content was deter- sintered glass filter at the bottom,through which pre- mined by using the method described by Mizukami et al. humidified air was supplied at a fixed rate of 0.5 vvm. (8).Its concentration was estimated as mg of acetyl About 40g of fresh cells (equivalent to 2g dry cell shikonin per liter of culture broth.An authentic sample of weight)precultivated in SHIK medium were harvested and acetyl shikonin was obtained from the Plant Cell Biology transferred asceptically to the reactor containing 1 of the Laboratory,Genetic Engineering Center,KIST,Taejon, same medium and 10 g of polyurethane cubes prepared as Korea. above. The temperature was maintained at 25C by circulating constant-temperature water through the jacket.The work- RESULTS AND DISCUSSION ing liquid volume was kept at 1.0/and the column was Shake flask culture with free cells Figure 2 shows the covered with aluminum foil to ensure darkness during the time courses for sucrose consumption and accompanying reactor operation. cell growth with a free suspension culture of L Analytical methods Sugar concentration was deter- erythrorhizon in SHCK and SHIK media.In SHCK me- mined by the DNS method (7). dium,cell growth reached up to 19g DCW//(450g To determine the cell mass with a free suspension culture FCW/after 10 d and sucrose was almost completely con- of the plant cells,the cells were first separated by using sumed at this point.Decrease of cell mass thereafter was stainless steel mesh (mesh size ca.30 um)and the fresh considered due to cell autolysis.Similar observations have cell weight(FCW)was measured from the filtered sample. been reported in other plant cell culture systems (9,10).It The dry cell weight(DCW)was then determined by drying was noted that cell growth was slightly retarded in SHIK the sample in an oven at 95C to a constant weight.For im- medium and reached up to 14g DCW//(330 g FCW/) after 14 d.With the cell line used in the present study,the shikonin produced was observed to be retained inside the cells during the culture period(Fig.3A),but its concentra- tion was negligibly small,as shown in Fig.2 Cell growth was seen to be closely related to the con- 40 20 30 (501X) 30 75 0 Q 云20 ● FIG.1.Schematic diagram of the packed-bed bioreactor system Time (d> with immobilized L.ervthrorhizon cells.I.Air flow meter,2.air FIG.2.Time courses of sucrose consumption,cell growth and filter,3.air humidifying vessel,4.sintered glass filter,5.sampling shikonin production with free cells of L.erythrorhizon in SHCK and port,6.water jacket,7.packed-bed of immobilized cells,8.inoculum SHIK media.Data points marked by circles and squares indicate port,9.antifoam inlet,10.condensor. those obtained from SHCK and SHIK media,respectively.318 PARK ET AL. J. FERMENT. BIOENG., erythrorhizon range in size from 50-300 pm, it was believed that the amount of polyurethane foam added provided enough space for the entrapment. Further incubation resulted in active proliferation of the entrapped cells, and the polyurethane foam cubes became fully packed with cell aggregates. Operation of bioreactor A packed-bed type bioreactor (Fig. 1) was employed for production of shikonin by using the immobilized plant cells. The internal diameter and height of the reactor were 80 and 300 mm, respectively. The column was made of a Pyrex glass tube with a sintered glass filter at the bottom, through which prehumidified air was supplied at a fixed rate of 0.5 vvm. About 40g of fresh cells (equivalent to 2g dry cell weight) precultivated in SHIK medium were harvested and transferred asceptically to the reactor containing 1 l of the same medium and 10 g of polyurethane cubes prepared as above. The temperature was maintained at 25°C by circulating constant-temperature water through the jacket. The working liquid volume was kept at 1.01 and the column was covered with aluminum foil to ensure darkness during the reactor operation. Analytical methods Sugar concentration was determined by the DNS method (7). To determine the cell mass with a free suspension culture of the plant cells, the cells were first separated by using stainless steel mesh (mesh size ca. 30/zm) and the fresh cell weight (FCW) was measured from the filtered sample. The dry cell weight (DCW) was then determined by drying the sample in an oven at 95°C to a constant weight. For ira- 11 :_.,. 10 8 dl = AIR 1 611 7 --'l 4 FIG. 1. Schematic diagram of the packed-bed bioreactor system with immobilized L. erythrorhizon cells. 1. Air flow meter, 2. air filter, 3. air humidifying vessel, 4. sintered glass filter, 5. sampling port, 6. water jacket, 7. packed-bed of immobilized cells, 8. inoculum port, 9. antifoam inlet, 10. condensor. mobilized cells, each sample of 20 cell-packed foam cubes was randomly taken from the culture broth. The cells were then easily separated from the polyurethane foam matrices by simple squeezing and washing. Cells collected in such a manner were then filtered, and the FCW and DCW of the sample were determined as above. Since shikonin is highly insoluble to water, and thus adsorbs well onto polyurethane matrices, loss of the product during cell separation is considered negligible. Shikonin was then extracted from the polyurethane foam cubes with chloroform and its content was determined by using the method described by Mizukami et al. (8). Its concentration was estimated as mg of acetyl shikonin per liter of culture broth. An authentic sample of acetyl shikonin was obtained from the Plant Cell Biology Laboratory, Genetic Engineering Center, KIST, Taejon, Korea. RESULTS AND DISCUSSION Shake flask culture with free cells Figure 2 shows the time courses for sucrose consumption and accompanying cell growth with a free suspension culture of L. erythrorhizon in SHCK and SHIK media. In SHCK medium, cell growth reached up to 19g DCW/I (450g FCW//) after 10 d and sucrose was almost completely consumed at this point. Decrease of cell mass thereafter was considered due to cell autolysis. Similar observations have been reported in other plant cell culture systems (9, 10). It was noted that cell growth was slightly retarded in SHIK medium and reached up to 14g DCW/I (330g FCW//) after 14 d. With the cell line used in the present study, the shikonin produced was observed to be retained inside the cells during the culture period (Fig. 3A), but its concentration was negligibly small, as shown in Fig. 2. Cell growth was seen to be closely related to the con- 40 ; ~ I00 ,3{ b~ .20+ I~ ° ° 20 ~ 10 25 0 - 0 4 8 12 16 0 Time (d) FIG. 2. Time courses of sucrose consumption, cell growth and shikonin production with free cells of L. erythrorhizon in SHCK and SHIK media. Data points marked by circles and squares indicate those obtained from SHCK and SHIK media, respectively