正在加载图片...
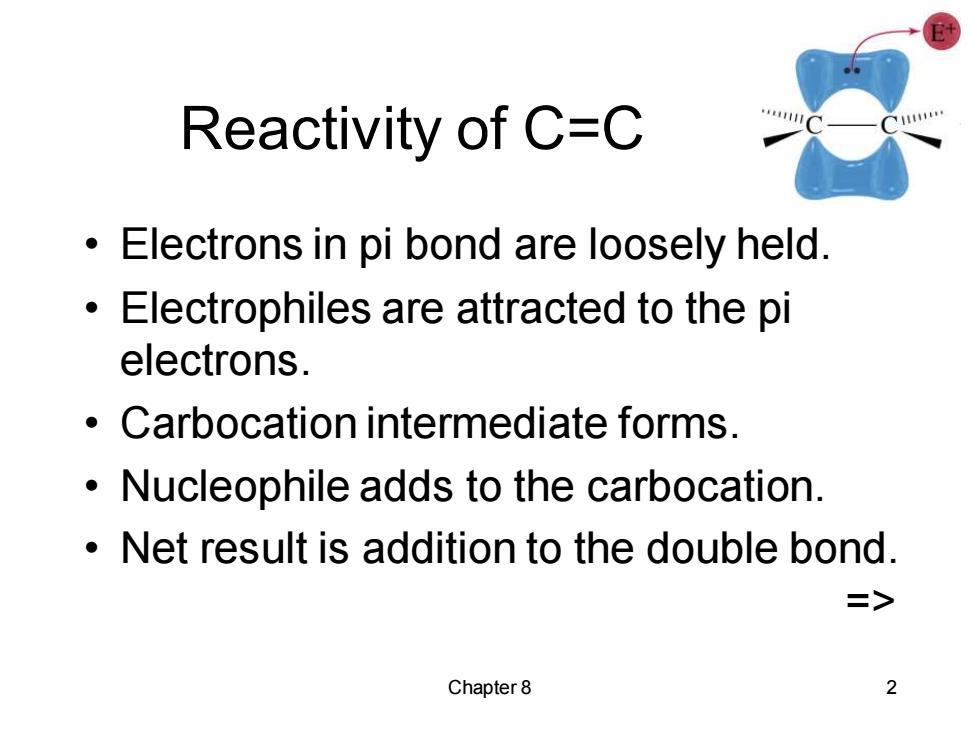
Reactivity of C=C Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. Carbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the double bond. 二> Chapter8 2Chapter 8 2 Reactivity of C=C • Electrons in pi bond are loosely held. • Electrophiles are attracted to the pi electrons. • Carbocation intermediate forms. • Nucleophile adds to the carbocation. • Net result is addition to the double bond. =>