正在加载图片...
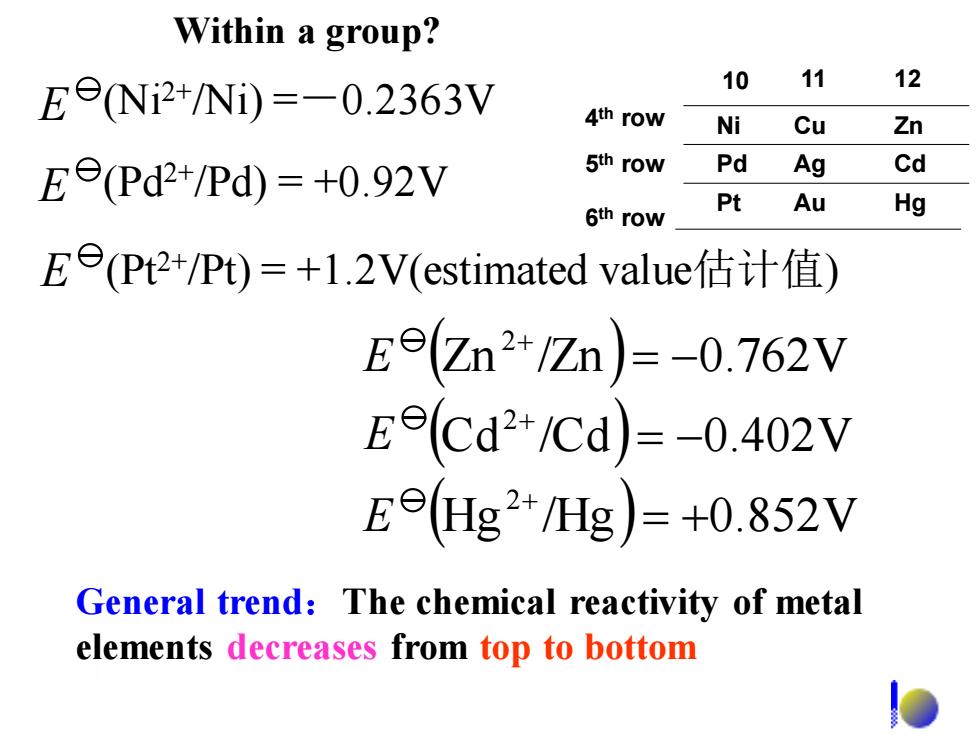
Within a group? E9Ni2+/Ni)=-0.2363V 10 11 12 4th row Ni Cu Zn Ee(Pd2+/Pd)=+0.92V 5th row Pd Ag Cd Pt 6th row Au Hg Ee(Pt2+/Pt)=+1.2V(estimated value估计值) Ee(Zn2+/Zn)=-0.762V Eecd2+/Cd)=-0.402V Ee(Hg2+/Hg)=+0.852V General trend:The chemical reactivity of metal elements decreases from top to bottomGeneral trend:The chemical reactivity of metal elements decreases from top to bottom ( ) ( ) (Hg /Hg ) 0.852V Cd /Cd 0.402V Zn /Zn 0.762V 2 2 2 = + = − = − + + + E E E E (Ni2+/Ni) =-0.2363V E (Pd2+/Pd) = +0.92V E (Pt2+/Pt) = +1.2V(estimated value估计值) Within a group? 4 th row 5 th row 6 th row Ni Pd Pt Cu Ag Au Zn Cd Hg 10 11 12