正在加载图片...
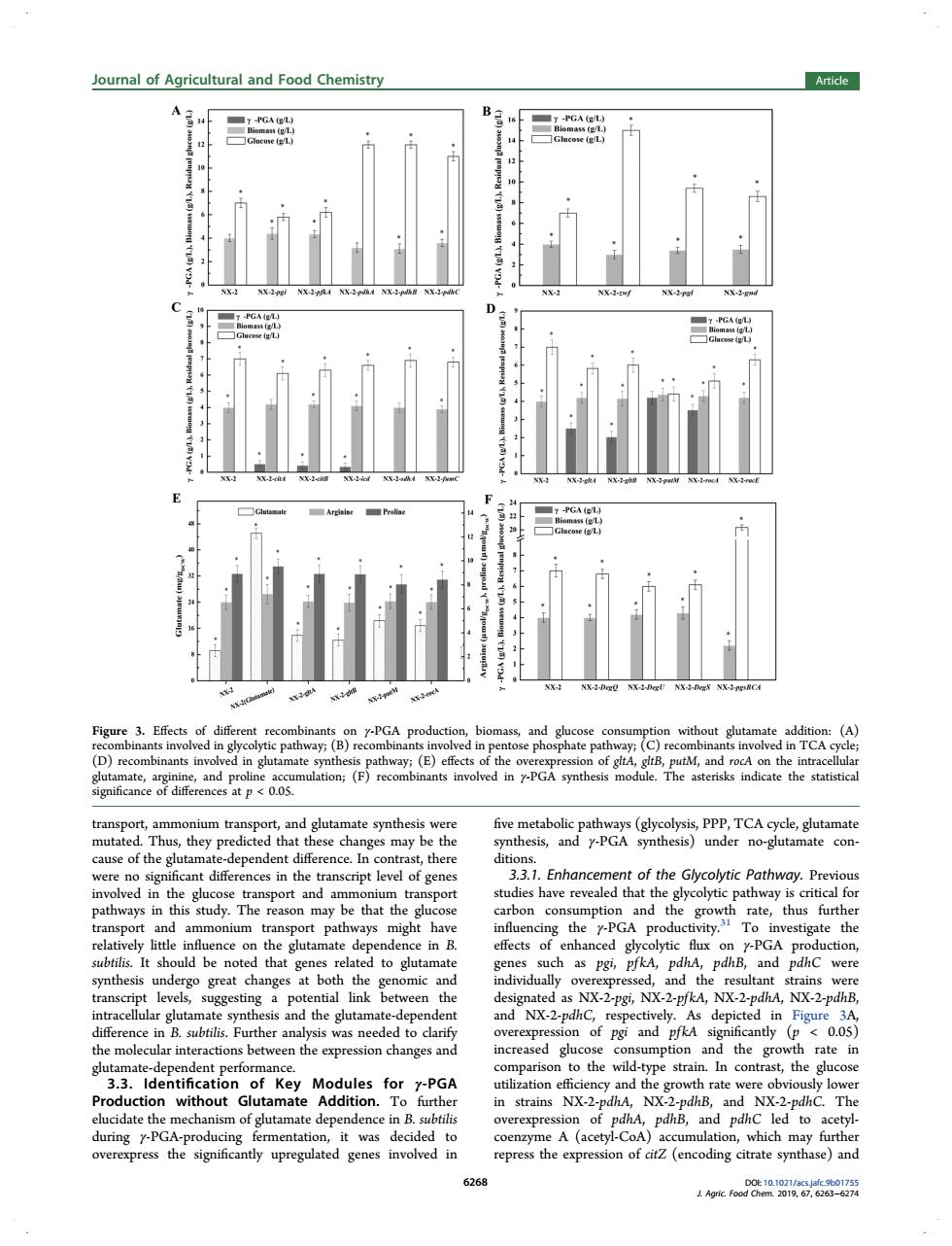
Journal of Agricultural and Food Chemistry Article Figure 3.Effects of dif re and glu : withoutgta nants in (a away:(E) of gitA PGA dule.The as ate synth five ay e the ause of the gl deper ent differenc n ,ther diti of ger eason may be that the on mption and the gro rate,thus further on 7-PGA p ge subtilis.It sh uld be note d that genes rela ed to glutamate genes pdhC were grea the nated as NX-2-pgi.NX-2-PfkA.NX-2-pdhA,NX-2-pdhB. sis and the glutamat increased glu tion and the h rate in the w contr the glu Modules PGA Production without Glutamate Addition. furthe in strains NX-2-pdhA. NX. -pdhB, NX-2 T overexpre of r-PG depende A repre the exp on of (encodin itat sythase)and 26 transport, ammonium transport, and glutamate synthesis were mutated. Thus, they predicted that these changes may be the cause of the glutamate-dependent difference. In contrast, there were no significant differences in the transcript level of genes involved in the glucose transport and ammonium transport pathways in this study. The reason may be that the glucose transport and ammonium transport pathways might have relatively little influence on the glutamate dependence in B. subtilis. It should be noted that genes related to glutamate synthesis undergo great changes at both the genomic and transcript levels, suggesting a potential link between the intracellular glutamate synthesis and the glutamate-dependent difference in B. subtilis. Further analysis was needed to clarify the molecular interactions between the expression changes and glutamate-dependent performance. 3.3. Identification of Key Modules for γ-PGA Production without Glutamate Addition. To further elucidate the mechanism of glutamate dependence in B. subtilis during γ-PGA-producing fermentation, it was decided to overexpress the significantly upregulated genes involved in five metabolic pathways (glycolysis, PPP, TCA cycle, glutamate synthesis, and γ-PGA synthesis) under no-glutamate conditions. 3.3.1. Enhancement of the Glycolytic Pathway. Previous studies have revealed that the glycolytic pathway is critical for carbon consumption and the growth rate, thus further influencing the γ-PGA productivity.31 To investigate the effects of enhanced glycolytic flux on γ-PGA production, genes such as pgi, pfkA, pdhA, pdhB, and pdhC were individually overexpressed, and the resultant strains were designated as NX-2-pgi, NX-2-pfkA, NX-2-pdhA, NX-2-pdhB, and NX-2-pdhC, respectively. As depicted in Figure 3A, overexpression of pgi and pfkA significantly (p < 0.05) increased glucose consumption and the growth rate in comparison to the wild-type strain. In contrast, the glucose utilization efficiency and the growth rate were obviously lower in strains NX-2-pdhA, NX-2-pdhB, and NX-2-pdhC. The overexpression of pdhA, pdhB, and pdhC led to acetylcoenzyme A (acetyl-CoA) accumulation, which may further repress the expression of citZ (encoding citrate synthase) and Figure 3. Effects of different recombinants on γ-PGA production, biomass, and glucose consumption without glutamate addition: (A) recombinants involved in glycolytic pathway; (B) recombinants involved in pentose phosphate pathway; (C) recombinants involved in TCA cycle; (D) recombinants involved in glutamate synthesis pathway; (E) effects of the overexpression of gltA, gltB, putM, and rocA on the intracellular glutamate, arginine, and proline accumulation; (F) recombinants involved in γ-PGA synthesis module. The asterisks indicate the statistical significance of differences at p < 0.05. Journal of Agricultural and Food Chemistry Article DOI: 10.1021/acs.jafc.9b01755 J. Agric. Food Chem. 2019, 67, 6263−6274 6268