正在加载图片...
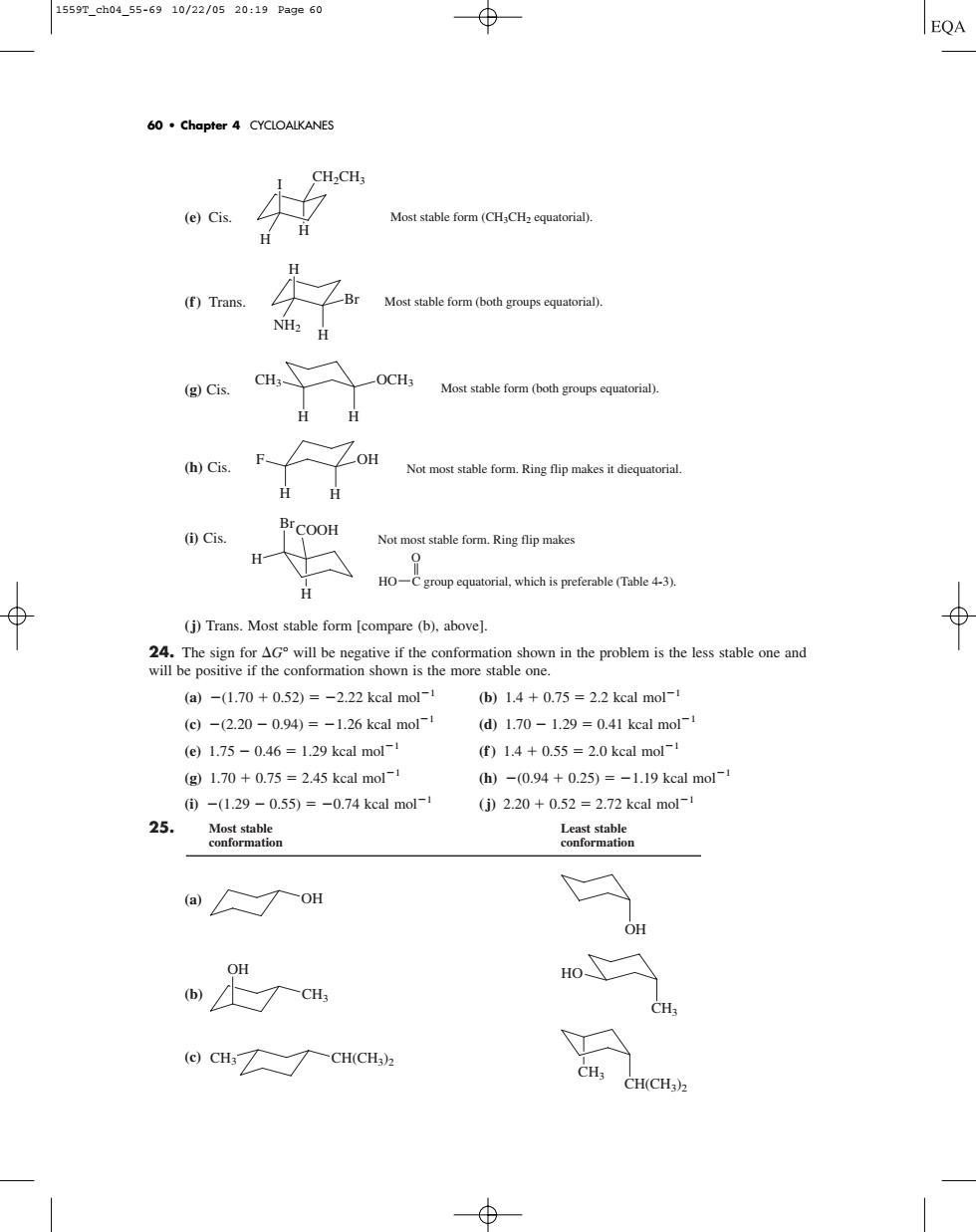
1559r_ch04.55-6910/22/0520:19Page60 EQA 60.Chapter 4 CYCLOALKANES (e)Cis. Most stable form (CH CHcqutoria). H (f)Trans Most stable form (both groupsqur). (g Cis. -OCH; Most stable form (oth group) Not most stable form.Ring flip makes it diequatorial ()Cis. Not most stable form.Ring flip makes HO-Cgroupqurial which is preferable (Table 4-3). (j)Trans.Most stable form [compare (b),above]. i the poblem is the le one and (a)-(1.70+0.52)=-2.22 kcal mol- b)14+0.75=2.2 kcal mol- (c)-(2.20-0.94)=-1.26 kcal mol (d1.70-1.29=0.41 kcal mol- (e)1.75-0.46=1.29 kcal mol- f)1.4+0.55=2.0 kcal mol (g1.70+0.75=2.45 kcal mol-1 h)-0.94+0.25)=-1.19 kcal mol- ①-(1.29-0.55)=-0.74 kcal mol-1 25 (j)2.20+0.52=2.72 kcal mol- ror (ao OH w尺a HO CH (CHCH(CH)(e) Cis. (f ) Trans. (g) Cis. (h) Cis. (i) Cis. (j) Trans. Most stable form [compare (b), above]. 24. The sign for G° will be negative if the conformation shown in the problem is the less stable one and will be positive if the conformation shown is the more stable one. (a) (1.70 0.52) 2.22 kcal mol1 (b) 1.4 0.75 2.2 kcal mol1 (c) (2.20 0.94) 1.26 kcal mol1 (d) 1.70 1.29 0.41 kcal mol1 (e) 1.75 0.46 1.29 kcal mol1 (f ) 1.4 0.55 2.0 kcal mol1 (g) 1.70 0.75 2.45 kcal mol1 (h) (0.94 0.25) 1.19 kcal mol1 (i) (1.29 0.55) 0.74 kcal mol1 (j) 2.20 0.52 2.72 kcal mol1 25. Most stable Least stable conformation conformation (a) (b) (c) CH(CH3)2 CH3 CH3 CH(CH3)2 HO CH3 CH3 OH OH OH COOH Br H H Not most stable form. Ring flip makes HO C group equatorial, which is preferable (Table 4-3). O F OH Not most stable form. Ring flip makes it diequatorial. H H CH3 OCH3 H Most stable form (both groups equatorial). H H Br H NH2 Most stable form (both groups equatorial). I H H Most stable form (CH3CH2 equatorial). CH2CH3 60 • Chapter 4 CYCLOALKANES 1559T_ch04_55-69 10/22/05 20:19 Page 60����������������